Applied Therapies: Antibacterials
1/169
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
170 Terms
What is the categorization of antibiotics for use in animals?
make by AMEG:
A: avoid, not authorized as vet meds in EU, no use in food animals, very limited use in companion
B: restrict, critically important in human med, only if no drugs in C or D
C: Caution, alternatives in human med, if no alternatives in D that would be clinically effective
D: Prudence, first line treatments
Which antimicrobial is now completely banned from use in animals in the EU?
Vancomycin (grouped with A but banned)
Which antimicrobials target the cell wall synthesis of organisms?
Beta Lactams: pencillins, cephalosporins
Vancomycin (banned in EU)
Bacitracin
What are beta lactam antimicrobials?
Beta Lactam ring (determines activity), weak acids, very WIDE safety margin, BACTERIOCIDAL, Spectrum: Gram positive→broad spectrum

What is the mechanism of action for the Beta-Lactams?
They target and inhibit transpeptidase (penicillin binding protein (PBP)) an enzyme that catalyses the formation of peptidoglycan polymers in the cell wall. Also bind additional PBPs in the cell membrane
Bactericidal effect = osmotically induced cell lysis
Time-dependence (T>MIC)→mechanism necessitates presence of drug throughout most of the dosing interval (want plasma levels to be greater than MIC for as long as possible)
What determines the susceptibility of bacterium to Beta-Lactams?
affinity for PBPs (bacterial species differences), ability to penetrate cell wall, ability to resist beta-lactamase enzymes
What are the 4 classes of penicillins?
Natural penicillins → Penicillin G, penicillin V
Beta-lactamase resistant penicillins (antistaphlococcal)→ methicillin, cloxacillin
Aminopenicillins→ amoxicillin, ampicillin
Extended-spectrum penicillins (antipseudomonal) → piperacillin, Ticarcillin
What is the basic structure common to all penicillins?
6 amino penicillanic acid (6-APA)
Where does Penicillin G come from?
Isolated by Alex Fleming in 1928, originally derived from Penicillium chrysogenum
AKA Benzyl Penicillin
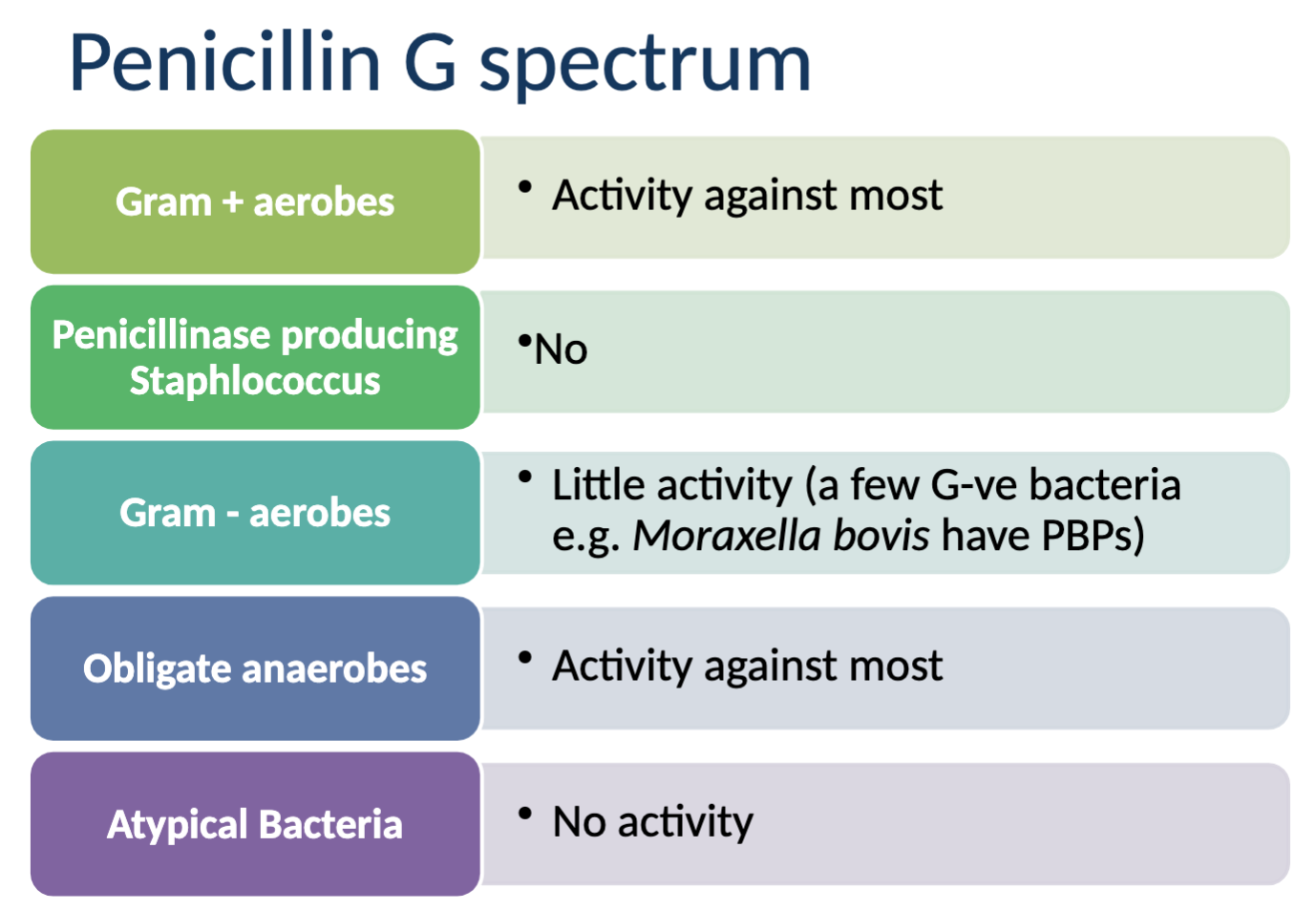
What are the characteristics of Penicillin G?
potent, highly bactericidal, time-dependent, category D antimicrobial, active in presence of pus and most organic matter
Activity against: most gram + aerobes, most obligate anaerobes, little activity against Gram - aerobes
NO activity agains: penicillinase producing Saph., atypical bacteria
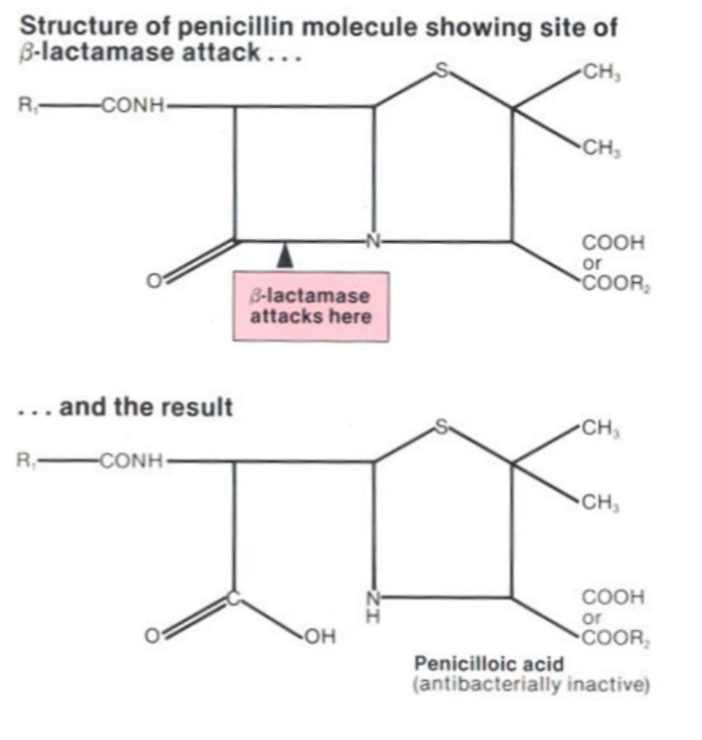
What are the limitations of Penicillin G?
Beta-lactam rings are fragile/unstable in presence of moisture (hydrolysis), pH/acid (degradation), high temp (degredation), enzymes like beta lactamase (degradation)
Forms of Penicillin G?
formulated/marketed for injection either as a crystalline powder for reconstitution (immediate use) or with excipients that protect structure
What are the pharmacokinetics of penicillin G?
absorption (IM) is rapid and complete (unless slow release formulation)
Distribution = effective plasma levels, diffuses freely to ECF, low lipophilicity (poor passage across cell membranes/limited diffusion to CNS unless inflamed), low Vd and not protein bound, short half life (T1/2 - 30 mins in dog)
Metabolism/elimination = excreted unchanged in urine (high concentration), active secretion gin proximal tubule
When is Penicillin G (Benzylpenicillin) used in a clinical setting?
drug of choice in treating infections in Gram + bacteria (strep., clostridia, corynebacteria, etc.)
one of the most frequently used drugs in large animal/equine med, often formulated in combo with aminoglycoside
How can the duration of Penicillin G effect be increased even when it has a short half life?
Time dependant with wide safety margin→
use higher doses, enables plasma levels to remain above MIC for longer
Intramuscular injection in depot form (slow acting, prolonged release) - anionic form of Pen G forms poorly water-soluble salts with substances cantaining a positively charge amino group (procane, benzathine, clemizole)
What are the drug interactions of Penicillin G?
(don’t mix with other in syringe - will inactivate)
Synergistic - aminoglycosides in vitro (facilitated entry of aminoglycodies), commonly marketed with streptomycin or in conjunction with gentamicin
What are the adverse effects of Penicillin G?
UNCOMMON
Hypersensitivity reactions, orally = may cause GI effects (anorexia, vomiting, diarrhea), neurotoxicity associated with very high doses or prolonged use, other effects like tachypnea/dyspnea/oedema/tachycardia have been reported
What are some dangers associated with depot preparations of Penicillin G?
MUST BE IM - IV WILL KILL
Procaine is prohibited in sport - don’t use in competing horses
toxic to small herbivores/pocket pets (may cause clostridial overgrowth)
Associated with abortion in cows
What are the therapeutic weaknesses of Penicillin G?
poor activity against Gram -s, destroyed by acid and beta lactamase, low volume of distribution, constitutive resistance/lack of action on targets w/in a broad range of microbes (narrow spectrum)
Which bacteria have beta lactamases?
Enterobacter, Escherichia, Klebsiella, Proteus, Pseudomonas, Staphlococcus
What are some characteristics of beta-lactamase resistant penicillin?
category D antibiotics with a narrow spectrum but effective against staph (Methicillin, oxacillin, cloxacillin, dicloxacillin)
active against: staph, variable against anaerobes
NO activity agains: gram negative
What are aminopenicillins?
developed in 1960s as broad-spectrum penicillins with enhanced gram - activity
considerable emerging resistance, sensitive to beta-lactamases
What are the clinical applications of aminopenicillins?
Category D, used for soft tissue infections in domestic species, CHEAP (Ampicillin, Amoxicillin)
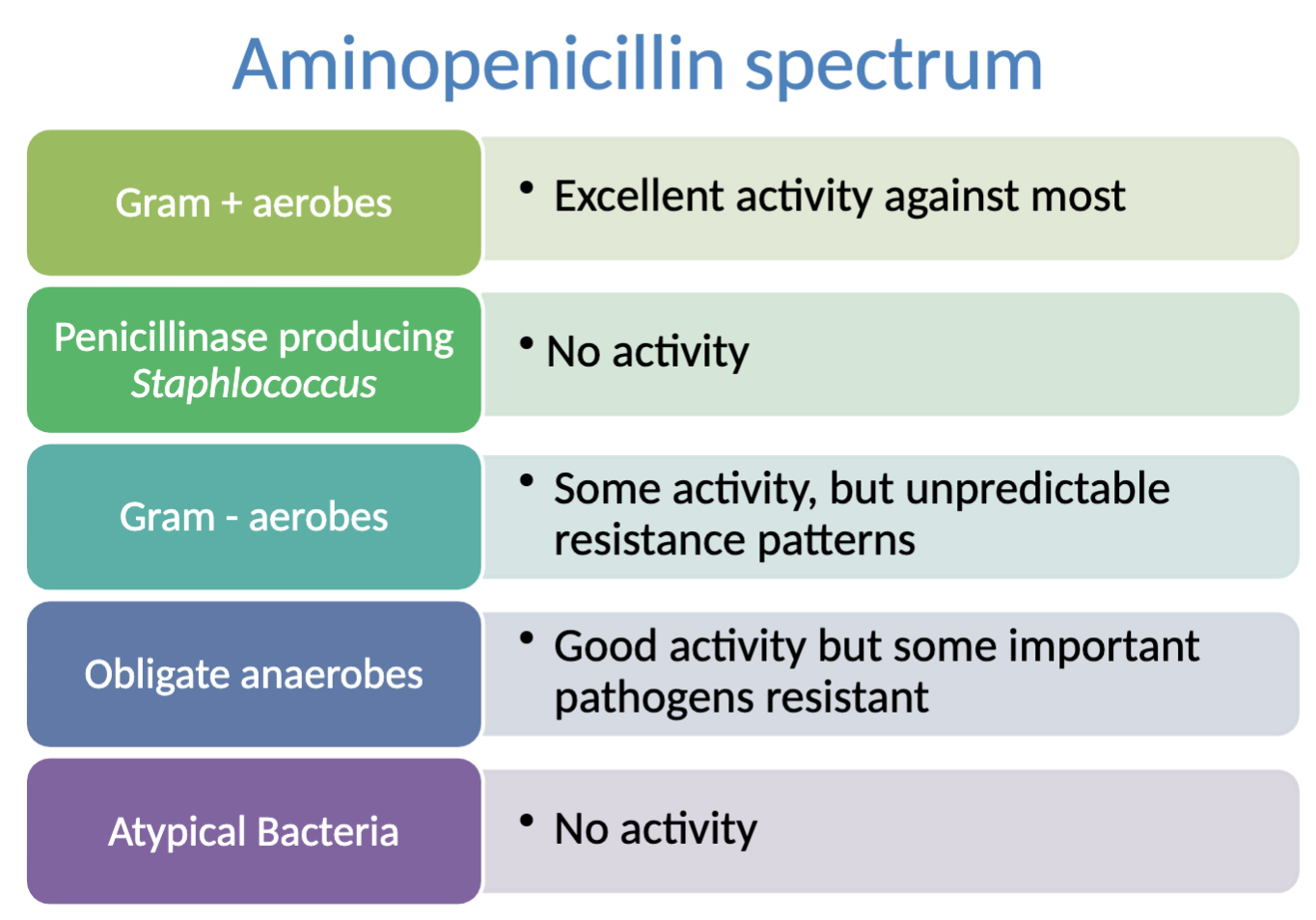
What is the activity spectrum of aminopenicillin?
activity against: most gram + aerobes, obligate anaerobes (some important pathogens RESISTANT), some activity against gram - aerobes (unpredictable resistance patterns)
NO activity against: Penicillinase producing Staph, atypical bacteria
How is amoxicillin ‘better’ than ampicillin?
more potent (lower MICs), more rapid bactericidal action, better oral bioavailability (2x more), better palatability, not adversely affected by food, longer t ½ greater Vd, better tissue distribution, more rapid clinical response
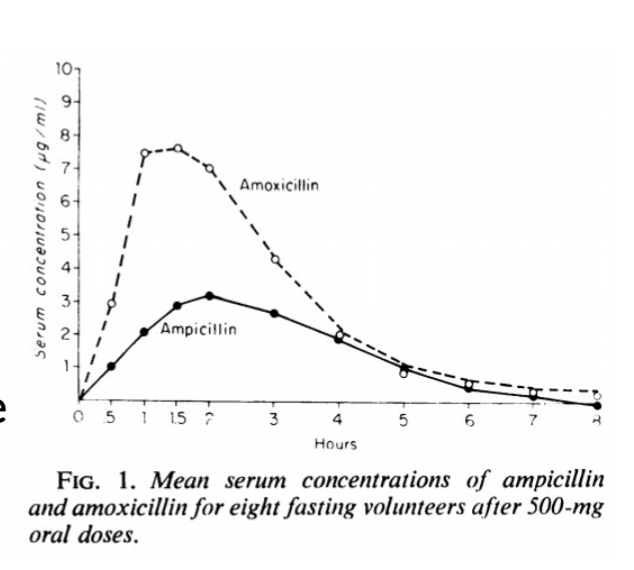
What is special/notable with the use of ampicillin and amoxicillin in horses and ruminants?
-oral formulations NOT used in horses (low bioavilability but foals have higher absorption rates-36-42%)
-microbial digestion means all beta-lactams have zero oral bioavailability in ruminants
What is a way to overcome beta-lactamase and how?
Clavulanic acid → bata-lactam ring similar to penicillin, fits the binding site of beta-lactamase
Acts by competitively and irreversibly binding to beta-lactamases and penicillinases produced by Staph.
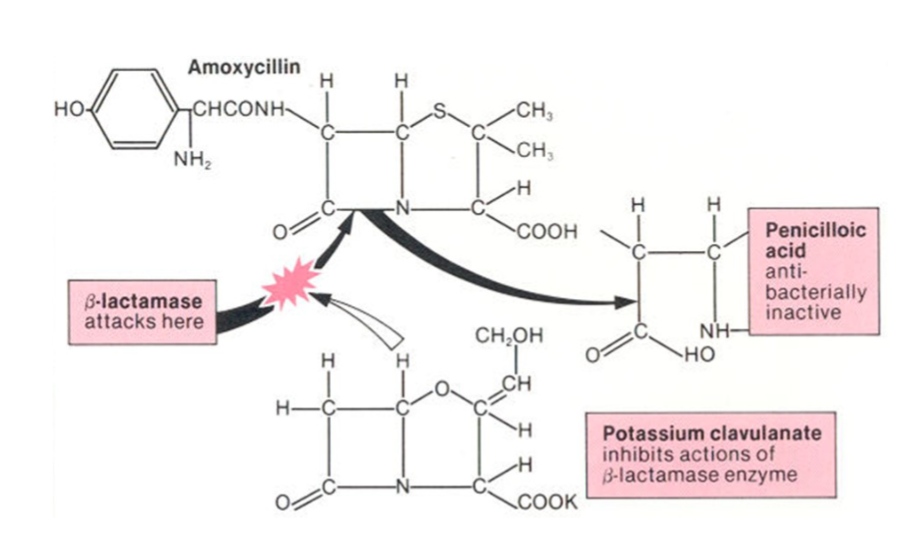
What are the characteristics of amoxicillin/Clavulanic acid?
Category C bactericidal aminopenicillin with beta-lactamase inhibitor combination (expands spectrum)
Clav acid -only weak antibacterial activity when used alone, available in fixed-dose combos with amoxicilin (oral) or ticarcillin (parenteral)
Ratio of 4:1 (ie. 200mg amoxi, 50mg clav)
What are the pharmacokinetics of Clavulanic acid?
well absorbed orally, bioavailability unaffected by ingesta, penetrates poorly into milk and CSF, T ½ shorter than amoxicillin
Affected by moisture →DRY SYRINGE ESSENTIAL, ‘storage’ in syringe not advisable
Excretion → glomerular filtration (high concentration in urine)
What is the activity spectrum of amoxicillin/Clavulanic acid?
Activity against: excellent → most Gram + aerobes, Penicillinase producing Staph (*MRSA resistant), obligate anaerobes; good → Gram - aerobes
NO activity against: Atypical Bacteria
What are extended spectrum penicillins (antipseudomonal)?
Category A - piperacillin-tazobactan ILLEGAL IN EU
effective against Pseudomonas spp. (gram - rod), susceptible to beta-lactamase
Primarily used for systemic and topical treatment (piperacillin-tazobactan)
What are characteristics of Cephalosporins/Cefalosporins?
Beta-lactam antibios from 1940s, isolated from Acremonium strictum spp from sewage
Structurally related to Penicillin G although cross resistance doesn’t necessarily occur
More resistant to beta-lactamases produced by Staph.
Can be more stable to pH and temp than Penicillin G, Broad spectrum, Bactericidal, Time-dependant antimicrobials
What/How does the Cephalosporin classification work?
“generations” - based mostly on pharmacokinetics and only partially on activity (1, 2, 3, 4), Ceph- (before 1975) and Cef- (after 1975)
What are the pharmacokinetics of cephalosporins?
Absorption - only a few are acid stable, not well absorbed, good bioavailability (75-90%) NOT IN HORSES
Distribution - distribute well except into CNS, even when meninges are inflamed (some exceptions)
Metabolism - varies depending on cephalosporin
Excretion - most T ½ relatively short (exception = cefovecin), most renally excreted
What are the clinical uses for cephalosporins?
used to treat soft tissue infections in a range of species (mastitis, metritis, urinary infection, skin, respiratory system, enteric-derived sepsis, bacterial conjunctivitis)
Osteomyelitis
What are the adverse reactions of Cephalosporins?
RELATIVELY UNCOMMON
Vomiting and diarrhoea in monogastrics, some risk of nephrotoxicity, rare bleeding disorders have been reported with some
What are some drug interactions of Cephalosporins?
Synergistic with aminoglycosides (same as penicillins)
Do not mix in syringe - will inactivate
SHOULD NOT BE USED WITH OTHER POTENTIALLY NEPHROTOXIC DRUGS
Characteristics of 1st generation cephalosporins?
Category C
Susceptible to beta-lactamase and cephalosporinases
Formulations: parenteral, oral, ophthalmic/intramammary/ intrauterine
What is the activity spectrum of 1st generation cephalosporins?
Activity against: excellent→Gram + aerobes, good→Penicillinase producing Staph (*MRSA resistant), moderate→gram - aerobes (not P. aeruginosa)
NO activity against: poor/unpredictable→obligate anaerobes, atypical bacteria
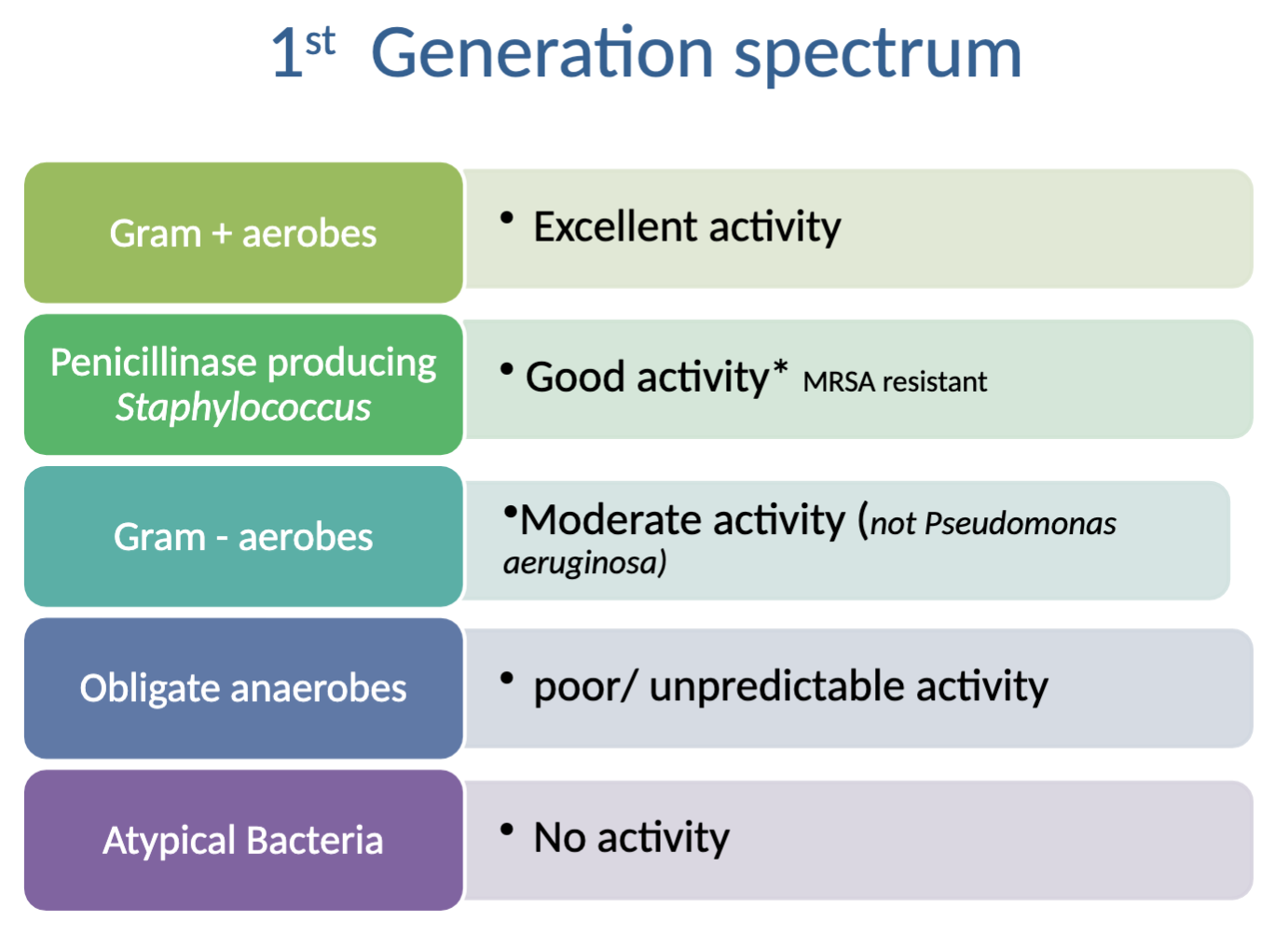
Why might you choose to prescribe a cephalosporin instead of amoxicillin in a soft tissue infection?
Greater activity against penicillinase-producing Staph (but NOT better than amoxi-clav combo) and generally greater activity against Pasteurella
**THE LAW says to choose something for than condition and species FIRST, even if amoxi-clav is available
Characteristics/activity spectrum of 2nd generation cephalosporins?
Category C
Formulations: parenteral
Differences from 1st = same efficacy against gram + pathogens but MORE EFFECTIVE in treatment of infections caused by Gram - bacteria (Enterobacter, E. coli, Klebsiella, and Proteus)
Example - Cefuroxime frequently used in surgical cases (as prophylaxis in GIT), can be given IV (data and doses available for dogs)
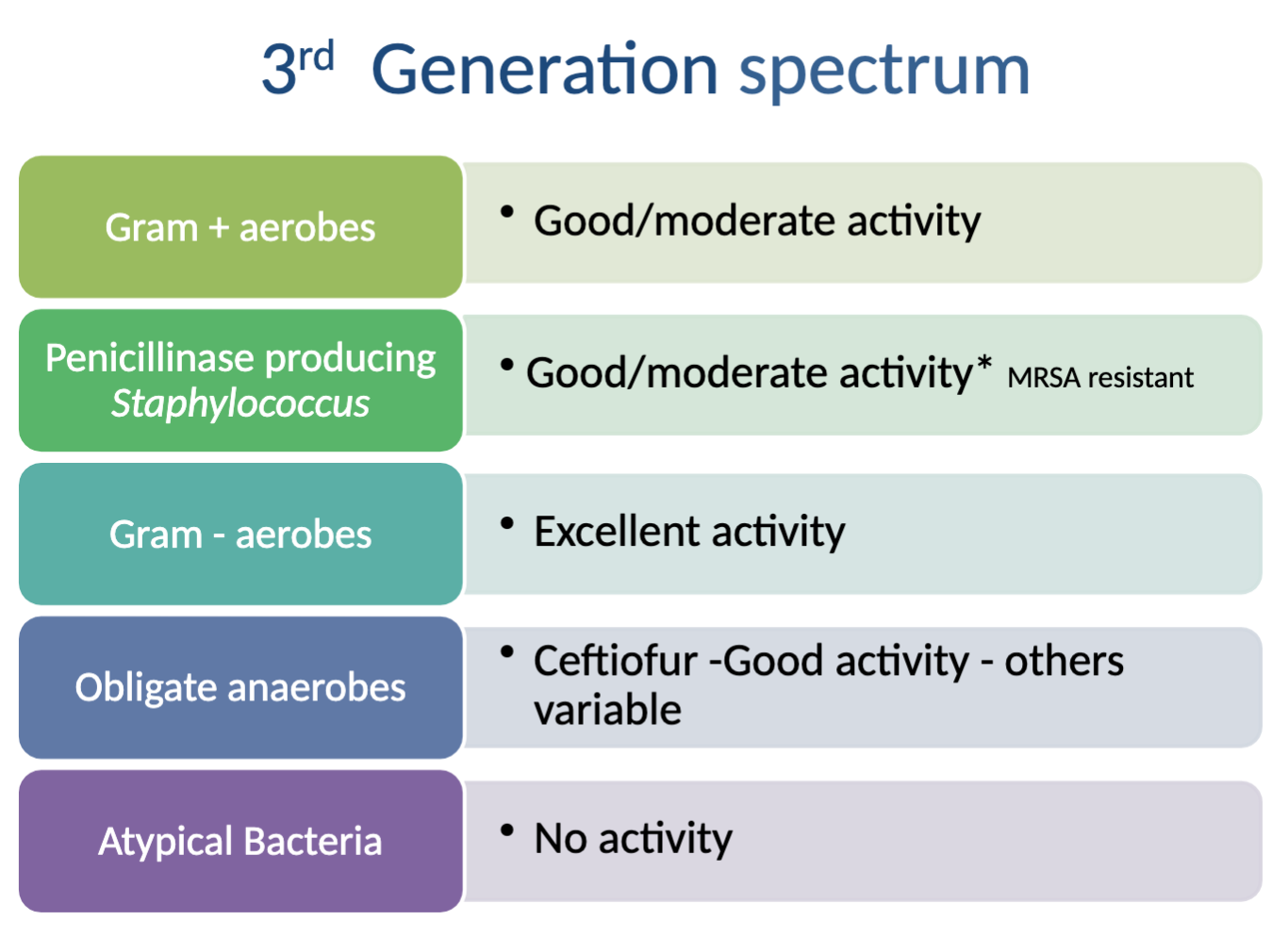
Characteristics/activity spectrum of 3rd generation cephalosporins?
Category B
Formulations: parenteral (good activity against Staph), oral (negligible against Staph), topical
Pros → most active of cephalosporins against Gram - aerobic organisms
Con→some members decreased activity against Gram + organisms
CAN CROSS BBB
What is the activity spectrum of 3rd generation cephalosporins?
Activity against: excellent→ Gram - aerobes, good/moderate→ Gram +aerobes, penicillinase producing Staph (MRSA resistant), Obligate anaerobes (CEFTIOFUR ONLY, others variable)
NO activity against: Atypical Bacteria
What is an example of Ceftiofur (3rd generation cephalosporins)?
Most common antibiotic used in dairy herds in North America
Excenel RTY - ceftiofur SQ →ZERO milk withdrawl (economical)
used in lactating dairy cows for treatment of foot lameness, metritis, respiratory infections, joint infections
What is an example of “long acting” Ceftiofur (3rd generation cephalosporins)?
Naxcel - injected into fat pad base of ear, useful for 7 days
AVOID INTRA-AURICULAR ARTERY INJECTION!!! death
What is an example of Cefovecin (3rd generation cephalosporins) use in companion animals?
Convenia → broad spectrum, aqueous solution (1ml/10kg)
Long T½ (long duration of action in dogs and cats)
>99% protein bound in cats and >98% in dogs, site of infection is important consideration (high plasma levels doesn’t mean it’ll reach infection outside of walls)
Used for skin infections and UTI in dogs and cats as a SQ injection 1x every 14 days (8mg/kg)
Freeze-dried power reconstituted with water → gradually discolours but doesn’t affect use. Must be refrigerated and discard after 56 days
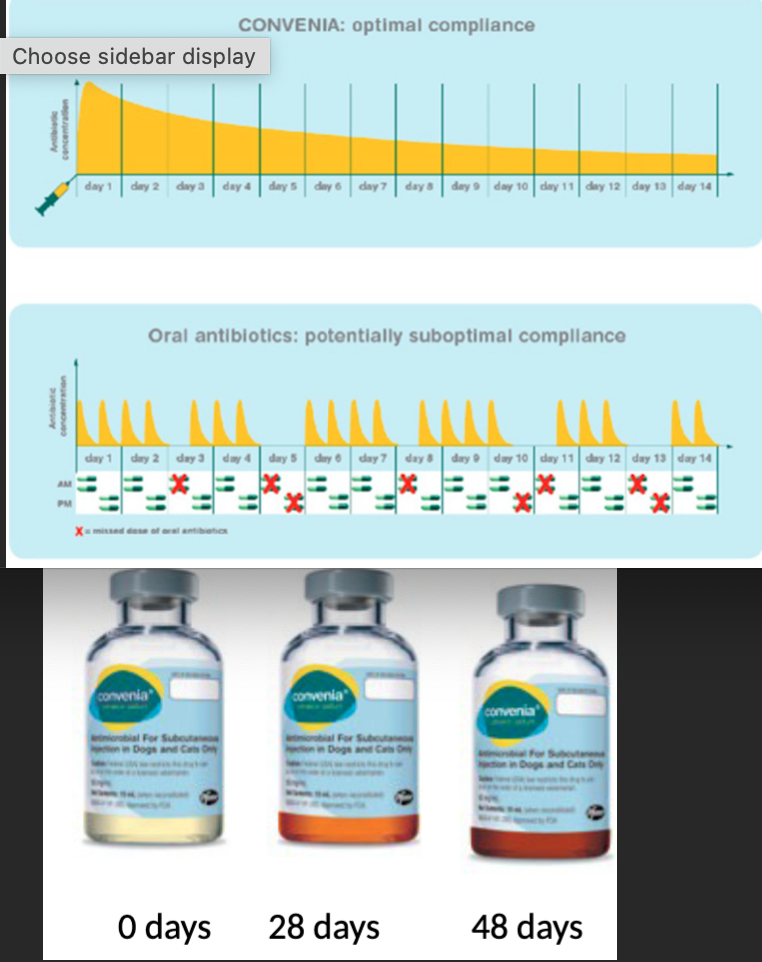
Why does convenia have low effectiveness against systemic (non UTI) E.coli or other Enterobacterales infections?
LIMITED due to high protein binding and inability to reach adequate drug concentrations for an adequate period
What are some Cefovecin (3rd generation cephalosporins) adverse effects?
Gastrointestinal - vomiting/diarrhoea, lethargy, anorexia (keep in mind, no take backs with injections)
Penicillin-like anaphylactic reactions require long-duration treatment due to long clearance time
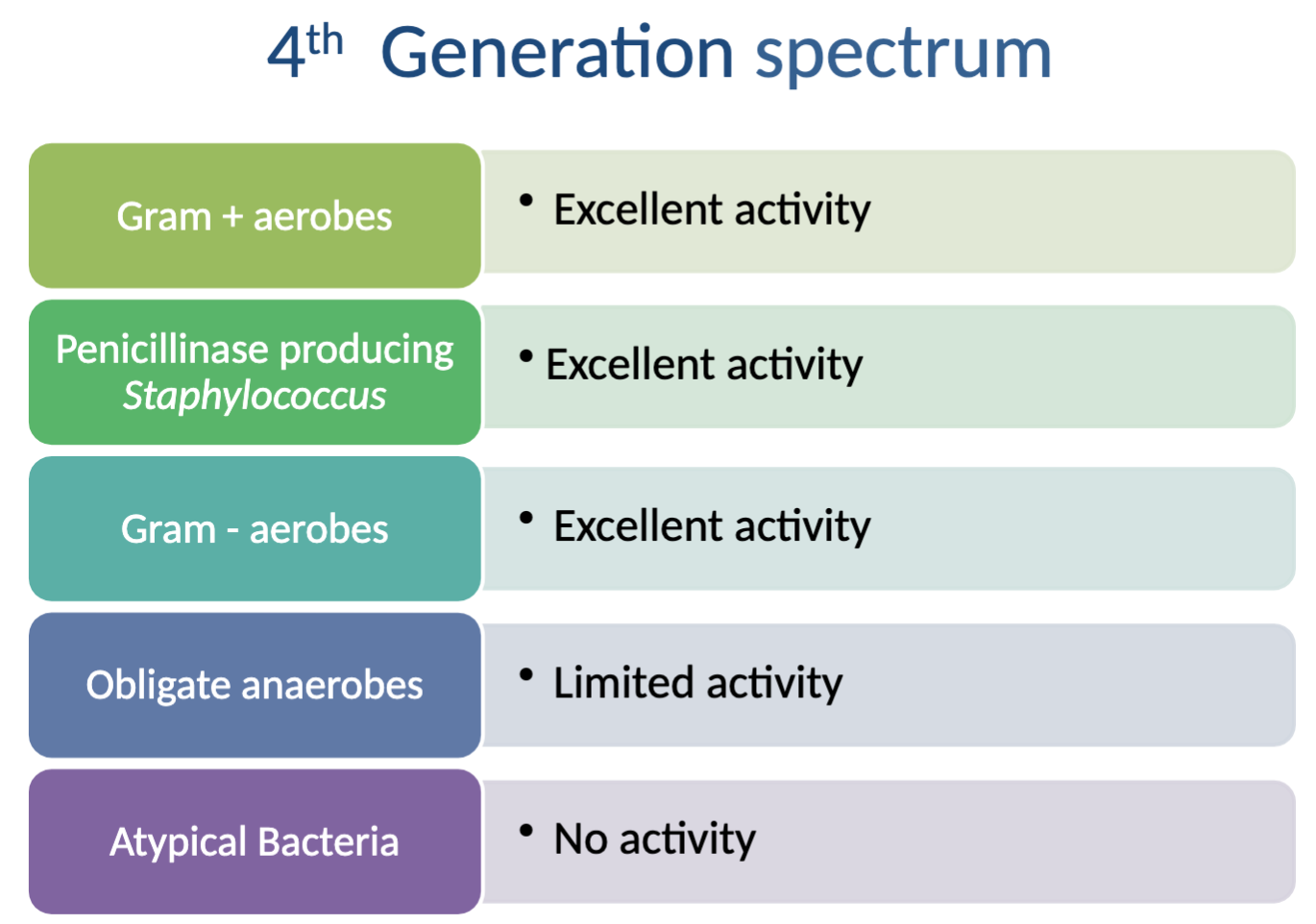
Characteristics/activity spectrum of 4th generation cephalosporins?
Category B, considered to be of particular value in human therapeutics
-retain excellent gram - activity (similar to 1st generation)
-retain excellent gram + activity (including Beta lactamase)
-high potency, low MIC, very little resistance seen
What is the activity spectrum of 4th generation cephalosporins?
Activity against: excellent→Gram + aerobes, penicillinase producing Staph (INCLUDING MRSA), Gram - aerobes, limited→ obligate anaerobes
NO activity against: Atypical bacteria
What is an example of a 4th generation Cephalosporin?
Cefquinome (not authorized in USA) → Parenteral or intramammary
Poor oral bioavailability (no oral products), licensed in EU for pigs, cattle, and horses to treat respiratory diseases, arthritis, meningitis, or dermatitis
What are the characteristics of Cefquinome (4th generation cephalosporin)?
Quaternary ammonium ion
Zwitterion→penetrates biological membranes easily, including all bacterial walls, NOT AFFECTED by beta lactamase due to charged structure (unlike other cephalosporins)
-forms very tight binding with PBPs = rapid cell killing
-unaffected by pus/necrotic tissue
What are the clinical uses of Cefquinome (4th generation cephalosporin)?
Cobactan -i/mammary tube for mastitis
Cabactan -parenteral for treatment of digital dermatitis BUT not the best treatment (proper management and foot baths=better, NOT through the use of antimicrobials)
Is Cefquinome (4th generation cephalosporin) not approved in the USA?
YES
extra-label use of cephalosporins in major food-producing animals is prohibited by the FDA
What is the proper antimicrobial stewardship of 3rd and 4th generation cephalosporins?
DON’T USE AS FIRST-LINE TREATMENT
Only be reserved for treatment of clinical conditions that have responded poorly or are expected to respond poorly
otherwise only after sensitivity testing carried out
Characteristics/activity spectrum of 5th generation cephalosporins and beta-lactamase combinations?
NO vet authorized versions of certain 5th gen cephalosporins (including 3rd gen combinations with btea-lactamase inhibitors)
→reserved for humans
*may be used in companion animals under exceptional circumstances but NOT IN THE EU
USA and CANADA = cephalosporins prohibited for extra label use in cattle, swine, chickens, or turkeys
What are the characteristics of Vancomycin?
Category A - banned in EU for vet med
Complex glycopeptide that bind to precursors of the peptidoglycan layer in bacterial cell walls
Only member in vet med= vancomycin

What is the mechanism of Vancomycin?
Prevents cell wall synthesis by binding to D-ala-D-ala protion of the cell wall precursors and activating cell wall autolysis
→Time dependent killing with persistence
Acquired resistance uncommon as yet-developing in enterococci-considered serious human health concern
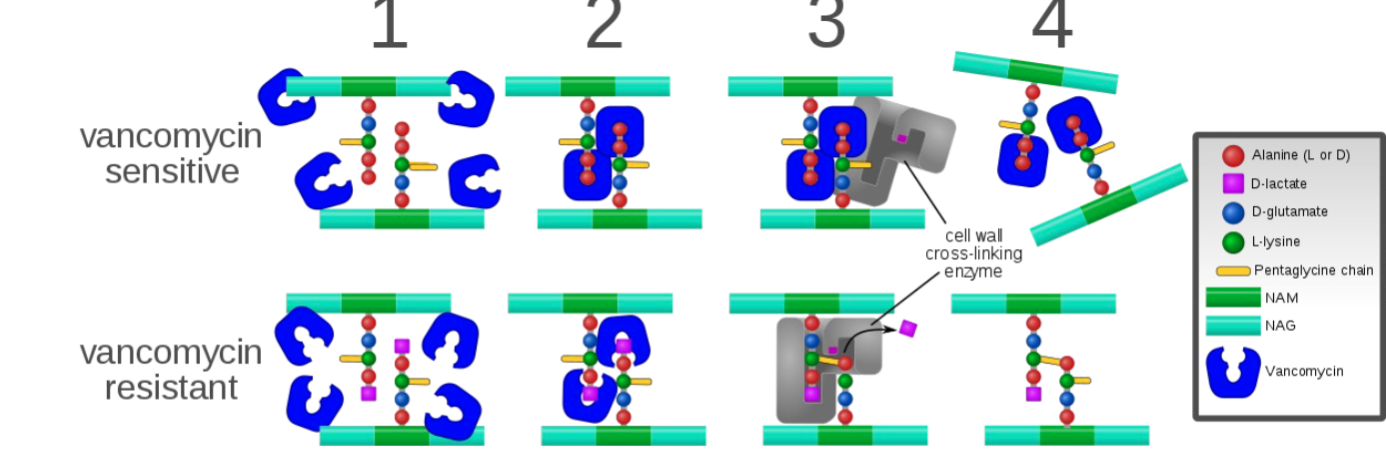
What is the activity spectrum of Vancomycin?
Activity against: excellent→Gram + aerobes, Penicillinase producing Staph. (INCLUDING MRSA), obligate anaerobes (gram + anaerobes)
NO activity against: Gram - aerobes, atypical bacteria
What are the pharmacokinetics of Vancomycin?
Poorly absorbed orally (PO) so must be given IV → normally pump infusion in dextrose of saline over 60 minutes as potential tissue damage
-penetrates tissues relatively poorly, short T½
Excretion = in active form via kidneys, dosage adjustment needed in renal patients
What are the adverse effects of Vancomycin?
Highly irritating so must be given slowly IV (if rapid, produces anaphylactic-type reaction), ototoxic in humans, potentially nephrotoxic
-Drug of LAST RESORT →only to be given in life threatening situations
What are the characteristics/mechanisms of Bacitracin?
Category D
Polypeptide product of Bacillus subtilis
Activity against Gram + bacteria →interferes with the transport of peptidoglycan precursors across the cytoplasmic membrane
NOT absorbed from GIT
Causes nephrotoxicity if given prenterally→use restricted to topicals (skin ointments and ear drops)
What are the characteristics/mechanisms of Polymixins?
Category B, Peptide
Surface active agents→disrupt cell membrane phospholipid and increase cell membrane permeability
-Gram - more sensitive than Gram +
-Active against P. Aeruginosa but NOT Proteus
-SERIOUS adverse effects = nephrotoxicity and ototoxicity of given parenterally os only used topically or opthalmically
What are the antimicrobials that target protein synthesis (30s)?
30s subunit: Tetracyclinges, Aminoglycosides
What are the antimicrobials that target protein synthesis (50s)?
50s subunit: Macrolides, Linocsamides, Amphenicols
Why are antimicrobials that target protein synthesis a little scary to use?
they target the bacterial ribosome and gene transcription, WITH the potential for affecting other mammalian cells!
What are the characteristics of Aminoglycosides?
Category C
Molecule composed of amino-modified sugars, often used as first line EVEN THOUGH category C
ALL inhibit bacterial protein synthesis, most bactericidal (depends on concentration), concentration dependent antimicrobial with potential for SEVERE adverse effects
products from the Steptomyces genus = -mycin
products from Micromonospora genus = -micin
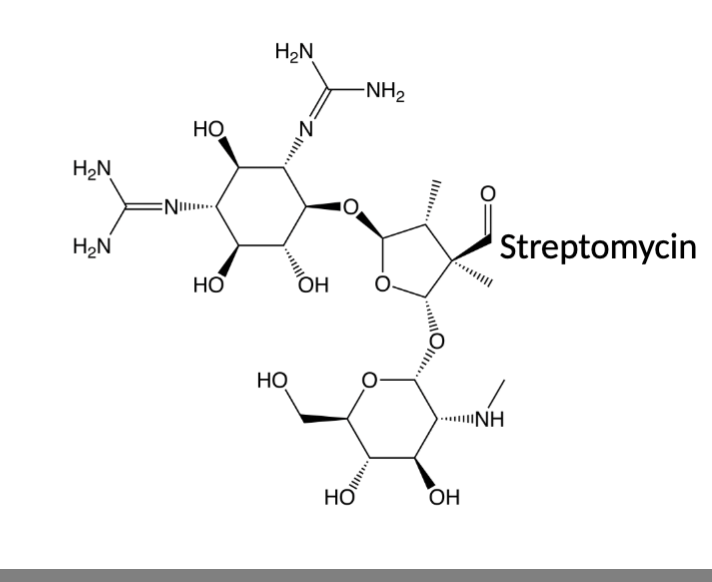
What was the first discovered Aminoglycoside?
First: Streptomycin (1943) as anti-tubercular drug, listed as a critically important antimicrobial (important in human gram - infections, especially multi-drug resistant ones)
What is the mechanism for Aminoglycosides?
Enter susceptible bacteria by oxygen dependent active transport (anaerobes are impervious to aminoglycosides), bind to S30 subunit of bacterial ribosomes, misreading of genetic code on mRNA, incorporation of incorrect AAs = irreversible inhibition of protein synthesis and rapidly bactericidal
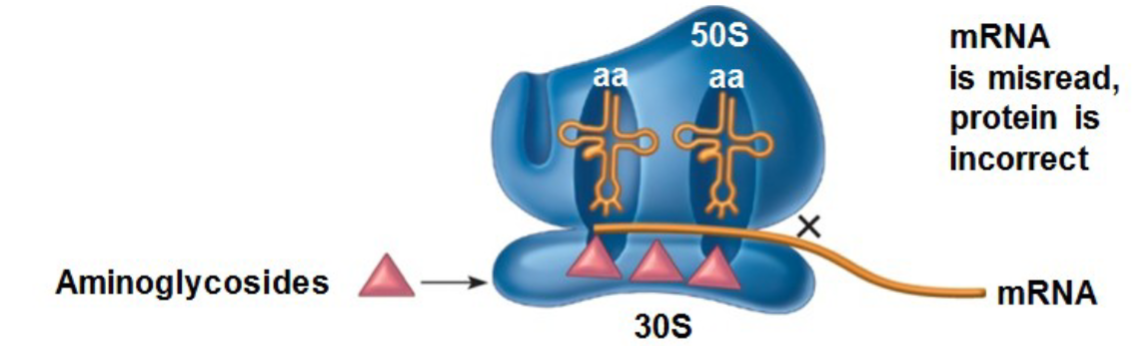
What is the spectrum of activity for aminoglycosides?
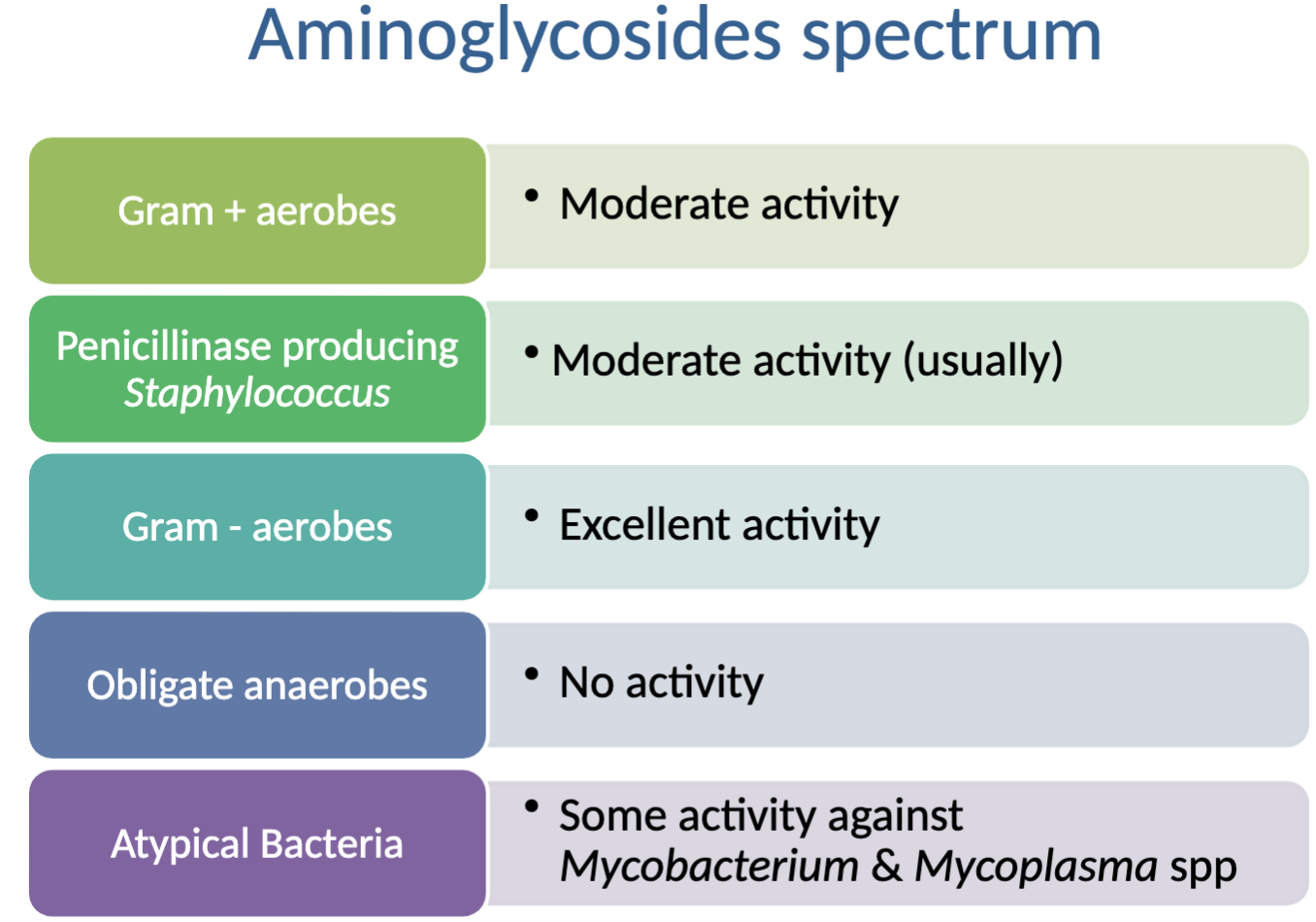
Activity against: excellent→Gram - aerobes; moderate→Gram +aerobes, penicillinase producing Staph. (usually); some→ atypical bacteria (Mycobacterium and Mycoplasma spp)
NO activity against: obligate anaerobes
What are the pharmacokinetics of aminoglycosides?
Poor oral availability (3-5%) in all species, polar, low lipophilicity, water soluble weak bases = low volume of distribution
T½ short (40-60mins) BUT persist in renal tissue for far longer than measured in plasma
Can enter synovia/pleural fluid (esp. if inflamed), therapeutic concentrations NOT achieved in brain/spine/prostate/milk
Acidic environment may decrease effectiveness →inactivated by pus (binds to nucleic acids in the debris of ruptured cells instead of bacteria)
Clearance/Excretion: glomerular filtration (affected by renal disease)
What is important about the prolonged post-antibiotic effect (PAE) with aminoglycosides?
Plasma levels do not need to continuously exceed MIC
-greater efficacy when given as a single large dose than in multiple smaller doses (despite short T½, once injection daily
What are some clinical applications of aminoglycosides?
Treats wide range of infections, particularly Gram - (Salmonella, P. aeruginosa, E.coli)
Pseudomonas in small animals and Gram - in horses/large animal
Commonly used in combo with beta-lactams (injection/intramammaries)
Many countried have banned/restricted use in crops/food animals (USA, India, Australia)
Which antimicrobial has also been used on citrus and apple crops, contributing to the concern of resistance?
Aminoglycoside → streptomycin
What are the adverse effects of aminoglycosides?
nephrotoxicity - death of renal tubular cells as binds to brush border and accumulated in lysosomes to inhibit lysosomal phospholipase
Sublethal alterations in tubular reabsorption and renal vasculature effects (vasoconstriction of afferent arterioles)
Clinical observation = (kidney fails but urine still produced)
Otoxicity -drug dependant vestibulotoxic/cochleotoxic/both, enters hair cells through mechanotransduction channels and intitiates an active signalling pathways that leads to cell death, irreversible damage, cats more susceptible than dogs and not considered an issue in farmed species
Neuromuscular blockage - High doses = muscle weakness and respiratory arrest, can prolong effects of certain NMJ blockers = respiratory depression (horses and intubation!!), Myaesthenia gravis patients also susceptible
Cardiac - slow the heart if given IV
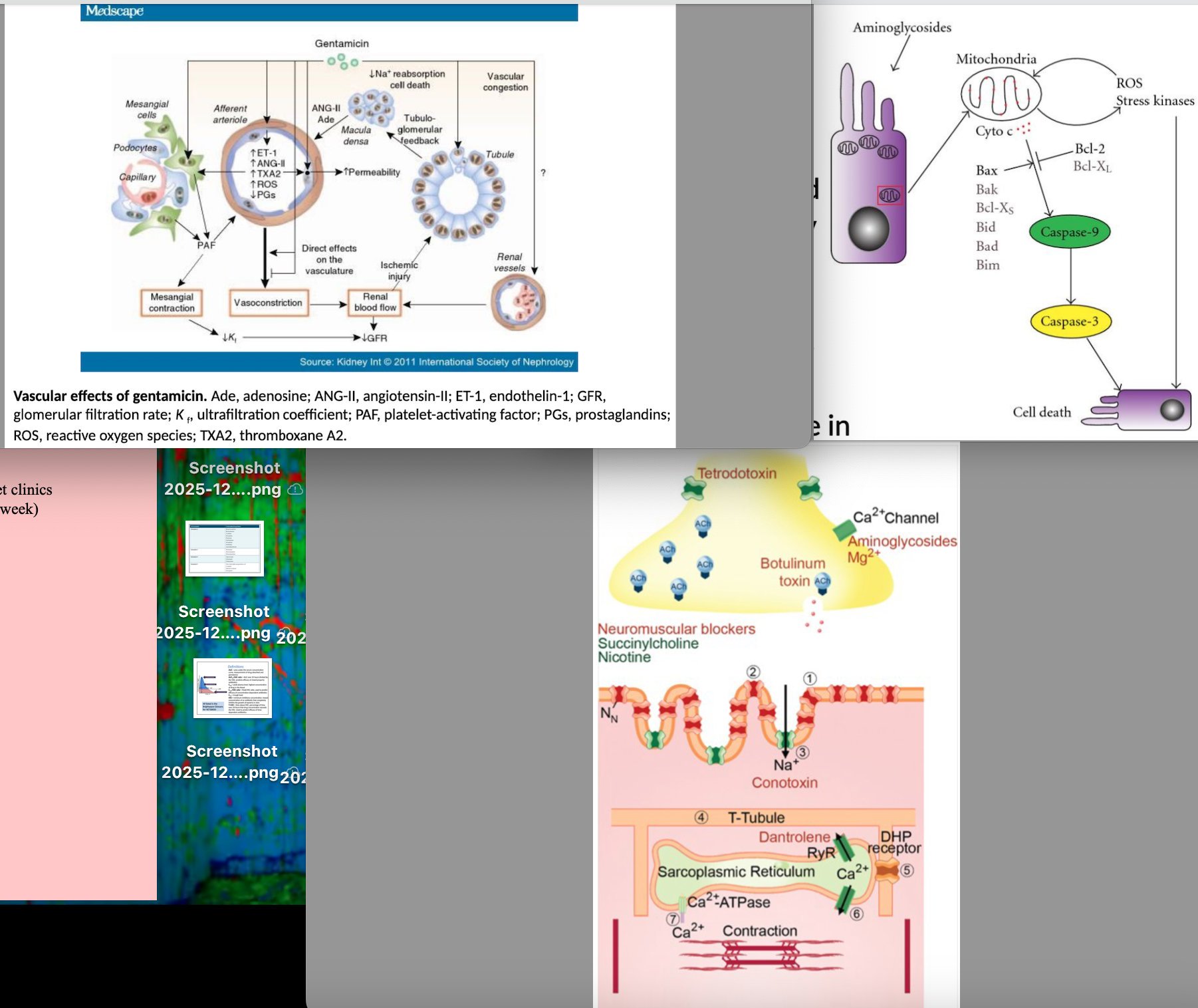
What are the factors that contribute to aminoglycoside nephrotoxicosis?
Total dose, duration of treatment, age (young/old particularly sensitive), renal function, dehydration and hypovolemia, aciduria, acidosis, hypomagnesemia, severe sepsis/endotoxemia, exposure to other potential nephrotoxic agents
What is a common example of an aminoglycoside?
Gentamicin
What are the characteristics of Gentamicin?
Category C
One of the most commonly used aminoglycosides for severe infections caused by Gram - bacteria in HORSES, may be more efficacious than other members against Staph. spp
Little activity against Mycobacterium
Formulations: Parenteral (injection), topical (eyedrops)
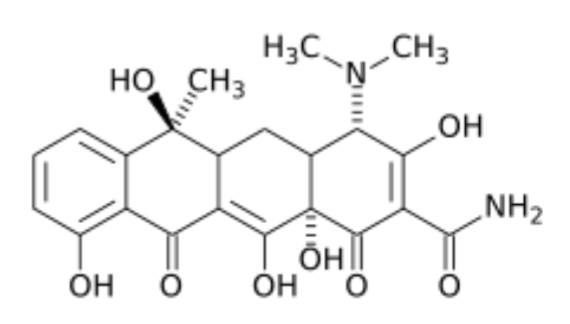
What are the characteristics of Tetracyclines?
Category D
First developed in 1940s, named for their 4 hydrocarbon rings
Bacteriostatic, time-dependant, and have persistent post-antibiotic effects (Parameter AUC24/MIC = best predictor of clinical efficacy)
Older, 1st generation = Chlortetracycline, Oxytetracycline (main in large), Tetracycline
Newer, 2nd generation = Doxycycline (main in small animals), Minocycline
What are the main tetracyclines used in large and small animal?
Large = Oxytetracycline
Small = Doxycycline
What is the best predictor of clinical efficacy of tetracyclines?
Parameter AUC24/MIC = best predictor of clinical efficacy
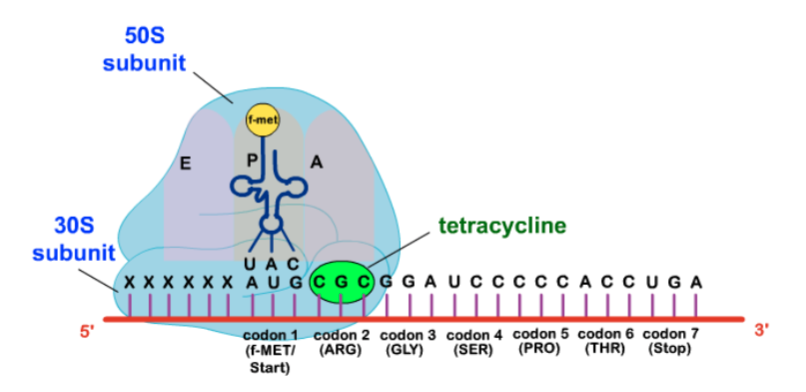
What is the mechanism of action of tetracyclines?
Passive diffusion through hydrophilic pores of outer membrane with energy dependant active transport through inner membrane (can be effective in anaerobic and hyperosmotic environments = works in anaerobes too!!)
-Bind to 30S subunit of bacterial ribosome and prevent protein synthesis by blocking the attachment of charged aminoacyl-tRNA to the A site on the ribosome
**ALSO binds to mammalian 80S ribosomal subunit = CAN INHIBIT PROTEIN SYNTHESIS IN HOST
What is the activity spectrum for Oxytetracycline (1st gen tetracycline)?
Activity against: good→gram + aerobes; moderate→Penicillinase producing Staph. (usually), Gram - aerobes, Obligate anaerobes; activity against Atypical bacteria (Rickettsia, Borrelia, Ehrlichia, and Mycoplasma)
NO activity against:
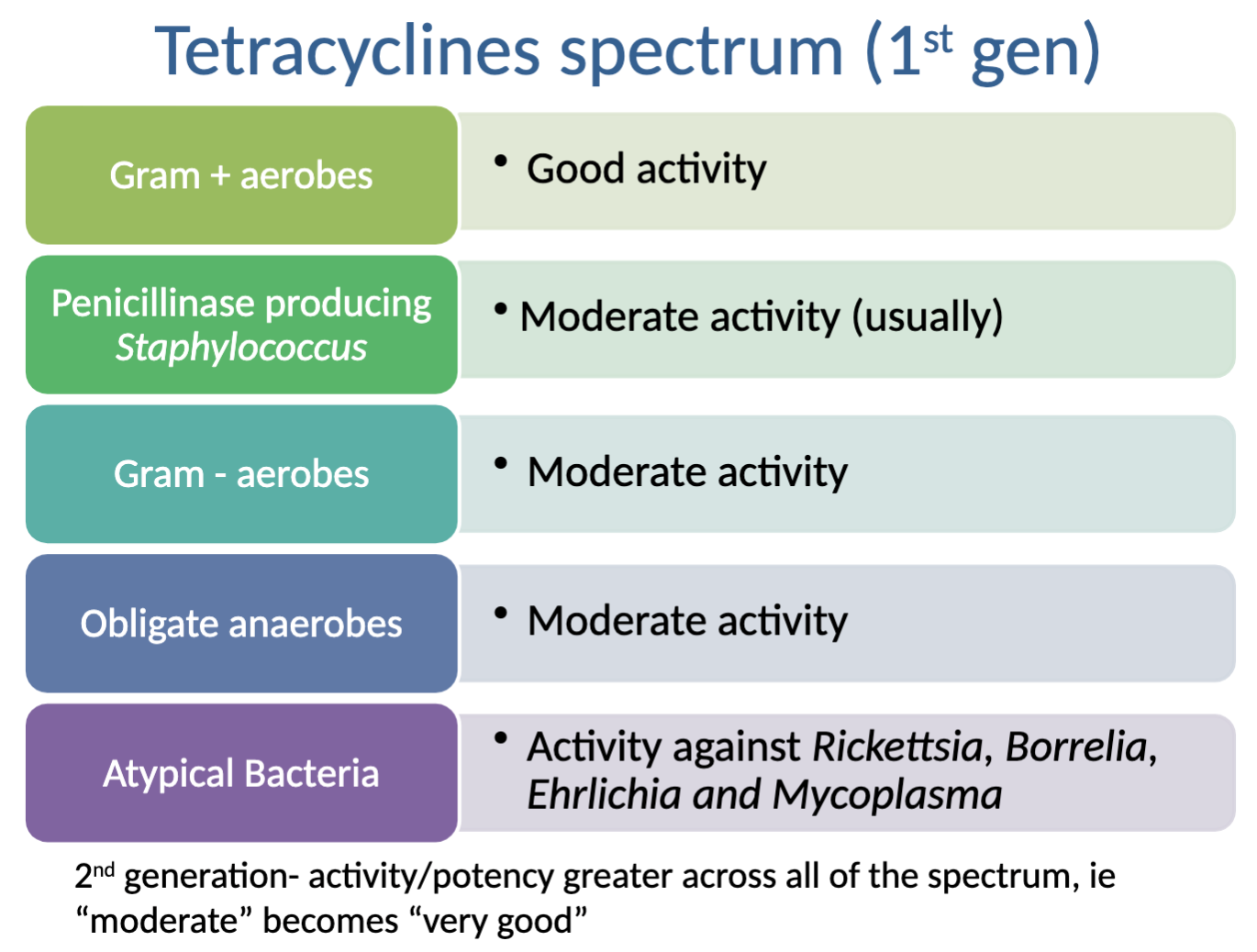
What is the activity spectrum for Doxycycline (2nd gen tetracycline)?
Activity against: Excellent→gram + aerobes; good→Penicillinase producing Staph. (usually), Gram - aerobes, Obligate anaerobes; activity against Atypical bacteria (Rickettsia, Borrelia, Ehrlichia, and Mycoplasma)
NO activity against:
What are some interesting chemical features of tetracyclines?
-Chelated easily (bind divalent ions like Ca++, Fe++) = decreases absorption esp. in dairy products = wrapping in cheese decreases absorption
-Concentrated in bone and teeth→can cause bright yellow discolouration in young animals (older tetras)
-Vary in lipid solubility = determines distribution/rate of excretion →Doxy. and minocycline are more lipophilic = better orally absorbed compared to others

How are tetracyclines given in large and small animals and why?
Following injection (sheep and cattle): Peak plasma levels within 2-6hrs, therapeutic levels maintained for up to 24hrs
Injection NOT recommended in horses or pets due to pain/irritation at site and erratic absorption→given orally instead, variable oral bioavailability (depends on drug)
What are the pharmacokinetics of tetracyclines?
Distribute well, found in highest concentration in Kidney/liver/spleen/lung, deposited at active sites of ossification, can diffuse into CNS/cerebrospinal fluid (BUT may not be preferred treatment especially in calf)
Pass through bovine placenta and enter foetal circulation
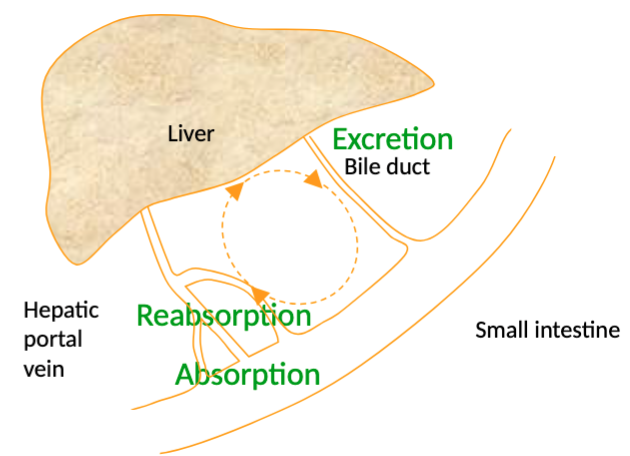
Elimination: tetracycline/Oxytetracycline excreted by kidney filtration (affected by renal function), DOXYCYCLINE removed via faeces (75% passive diffusion into intestinal lumen, 5% from bile), some enterohepatic recycling
****DOXY may be drug of choice in patients with impaired renal function!!!!
What are the normal clinical applications of Tetracyclines?
FARMED: general mixed infections as broad spectrum and cheap, used extensively particularly in bovine and porcine respiratory disease complex
COMPANION: atypical bacterial disease due to Chlamydophila (particularly cats), Ehrlichia canis & Rickettsia rickettsii & Mycoplasma infections
What are the special applications of Tetracyclines (some outside of marketing authorization/extra label)?
delineate tumours by fluorescence
Demethylchortetracycline used to inhibit action of antidiuretic hormone in cases of excessive water retention
Aid “stretching” of contracted flexor digital tendons in neonatal foals (due to metalloproteinase-inhibiting effects/binding of Ca)
Reduce risk of adverse events/enhance killing of adult heartworms and/or microfilaria before adulticide therapy
What are the adverse effects of tetracyclines?
GIT disturbances especially in cats (irritant, can cause vomiting), occasional fatal anaphylaxis, rapid IV injection can cause hypotension and collapse, severe liver damage reported after overdosage, contradicted in renal disease (except doxy-liver metabolism)
→dose related renal tubular damage (2-3x authorized/labelled), exacerbated by dehydration and other nephrotoxic drugs
Excessive accumulation in teeth/bone can reduce calcification (1st gen, important in show cattle), severe effects reported in guinea pigs/hamsters/other hindgut fermentors→ superinfection/enterocolitis (particularly horses)
Should not be used in pregnant animals due to anti-anabolic effects (depresses protein production)→anti-anabolic effects exacerbated by concurrent corticosteroids
What are some examples of large animal tetracyclines?
Oxytetracycline (“Oxytet” “Alamycin” “Engemycin” “Terramycin”)
Many products authorized in Ireland and more in USA (>120), used in broad range of species
Formulations: Topical spray (‘purple/blue’ spray), Parenteral (IV, IM, long acting), Oral (medicated feeds, tablets)
What are some common conditions in farmed animals that would indicate the use of Oxytetracyclines?
General mixed infections (broad spectrum and cheap, first line for many), BUT semi/not very effective against Mycoplasma bovis
Meningitis/neuro→commonly used for listeriosis in high concentrations BUT penicillin is preferred choice
Cellulitis, foot lameness
Metritis→*if cow very ill/immunosuppressed, a beta-lactam might be a better choice
What are some examples of companion animal tetracyclines?
Doxycycline hyclate → treatment of respiratory tract infections in cats/dogs, where “unusual bacteria” suspected in cats (ie. fever of unknown origin (FUO)), leptospirosis in dogs
Used as part of the therapeutic protocol in Heartworm (D. immitus)
**Doxy used in-feed/water for pre-ruminant calves, poultry and pigs (EU)
Is Doxycycline authorized in the USA?
NO! There is currently NO FDA authorized doxy tablets for vet use in USA
(must be prescribed extra-label)
What are the adverse effects specifically for Doxycycline?
VERY well absorbed orally BUT doxy tablets associated with oesophageal erosion and stricture in cats (ALWYAS give water)
What is important to do with doxycycline when dosing cats (or any companion animal honestly)?
AVOID DRY SWALLOW→ follow administration with 2-5ml of fluid! (local irritation that stays)
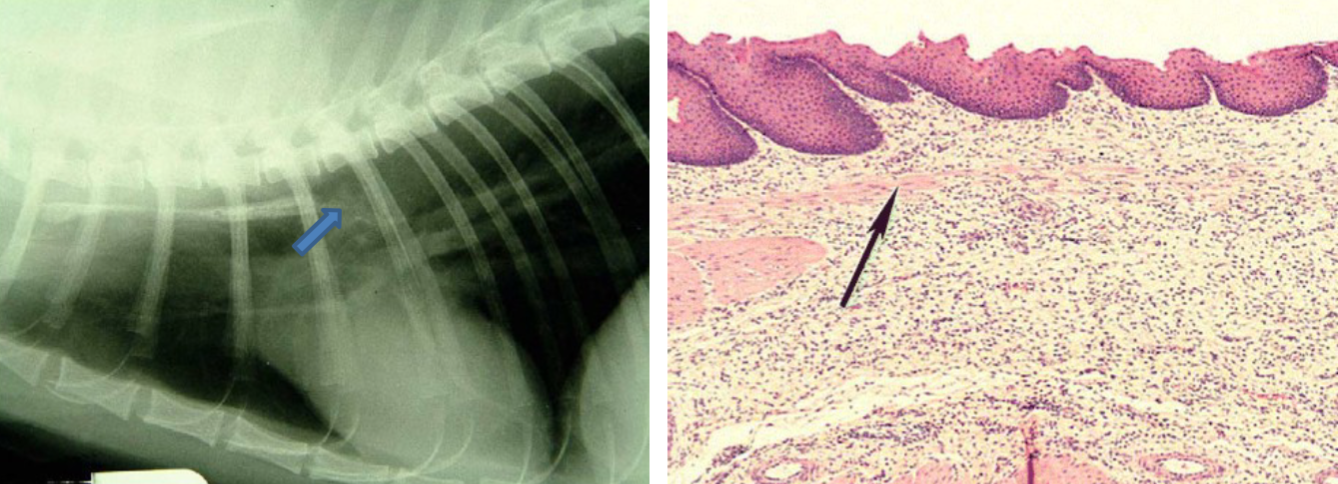
What are the antimicrobials that affect bacterial protein synthesis by targeting the S50 subunit?
Macrolides, Lincosamides, Amphenicols
What are the characteristics of Macrolides?
Category C
(first) Erythromycin→produced from Saccharopolyspora erthraeus, first discovered in 1952
Activity derives from large macrocyclic ring, Weak bases, high lipophilicity, Largely water insoluble and unstable in gastric acid

What is the mechanism of action for Macrolides?
Enter the cell and reversibly bind to the 50S ribosomal subunit, inhibiting translocation of peptides→inhibiting protein synthesis
Unable to cross mammalian mitochondrial membrane = DON’T produce bone marrow suppression (relatively safer)'
Debate: concentration vs time dependence but potential for time dependant (T>MIC) bactericidal action, particularly with high concentrations
PAE (post antibiotic effect) CAN be produced and duration is drug+pathogen dependent
Antimicrobial action can be enhanced by high pH (7.8-8) and suppressed by low pH →site of infections tend to be acidic
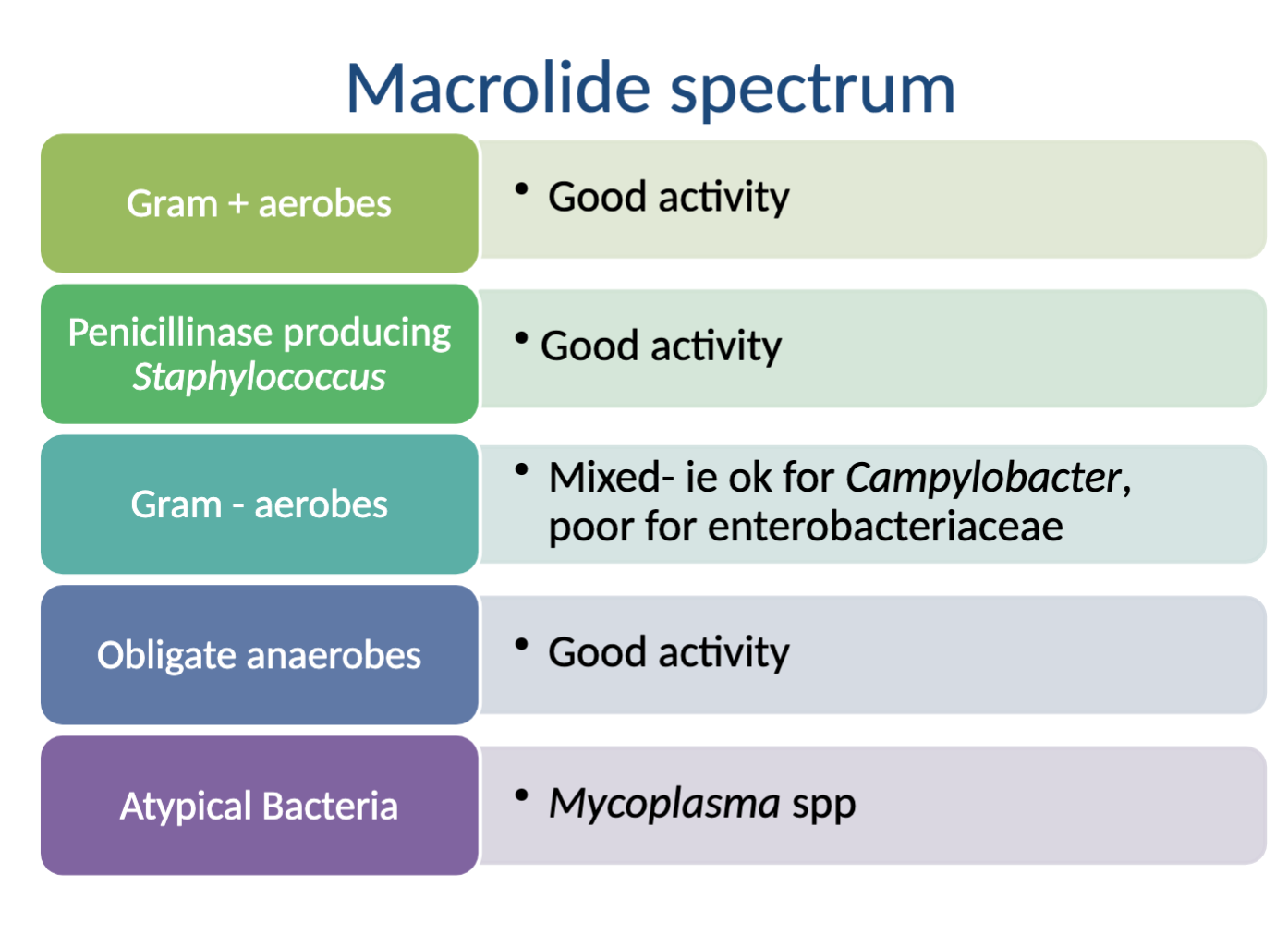
What is the spectrum of activity for Macrolides?
Very broad but not great
Activity against: good→Gram + aerobes, Penicillinase producing Staph., Obligate anaerobes, Atypical bacteria (Mycoplasma); mixed/ok→Gram - aerobes (Campylobacter)
NO activity against: poor→Gram - aerobes (enterobacteriaceae)