Redox Reactions: Oxidation, Reduction, and Electron Transfer in Chemistry
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
What is a redox reaction?
A type of chemical reaction that involves a transfer of electrons between two species.
What does oxidation refer to in a redox reaction?
The process where an atom loses electrons.
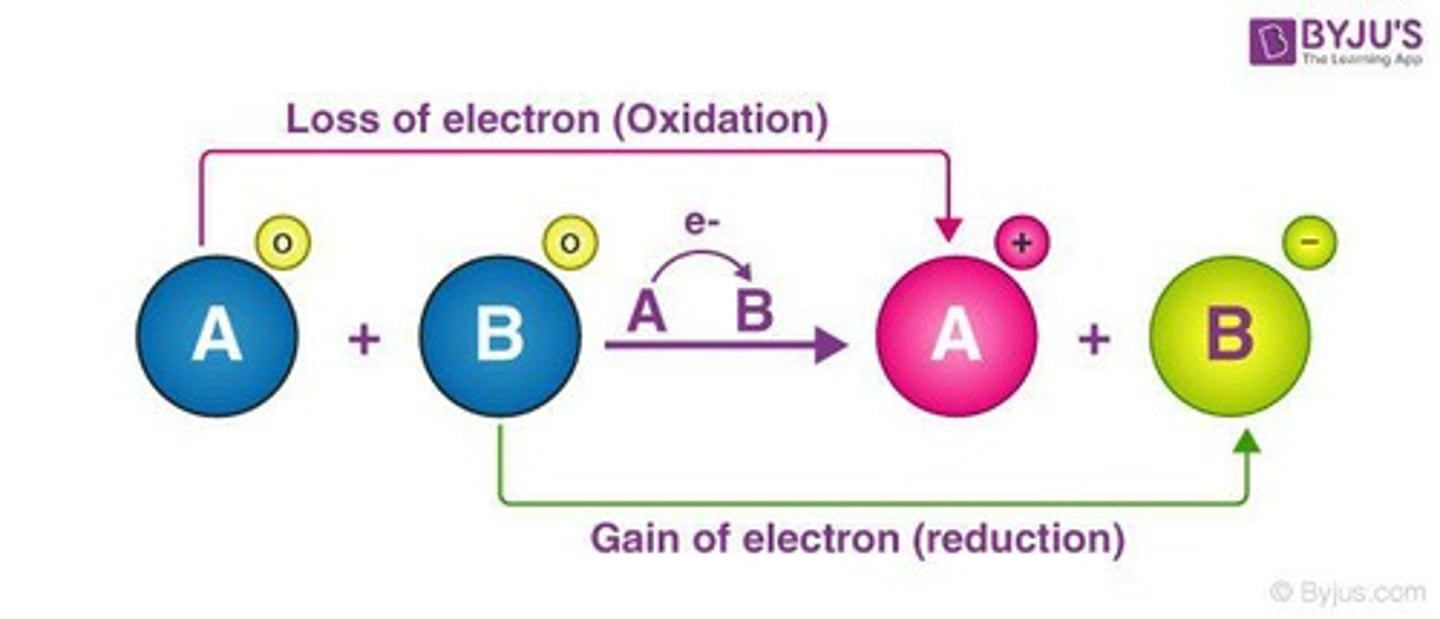
What does reduction refer to in a redox reaction?
The process where an atom gains electrons.
How can you identify which species is oxidized in a reaction?
By determining which species has an increase in oxidation number.
How can you identify which species is reduced in a reaction?
By determining which species has a decrease in oxidation number.
What is an oxidation number?
The number of electrons that an atom loses, gains, or appears to use when forming compounds.
What is the oxidation number of sodium (Na) in NaOH?
+1
What is the oxidation number of oxygen (O) in NaOH?
-2
In the reaction Zn + 2H+ → Zn2+ + H2, which element is oxidized?
Zinc (Zn) is oxidized from 0 to +2.
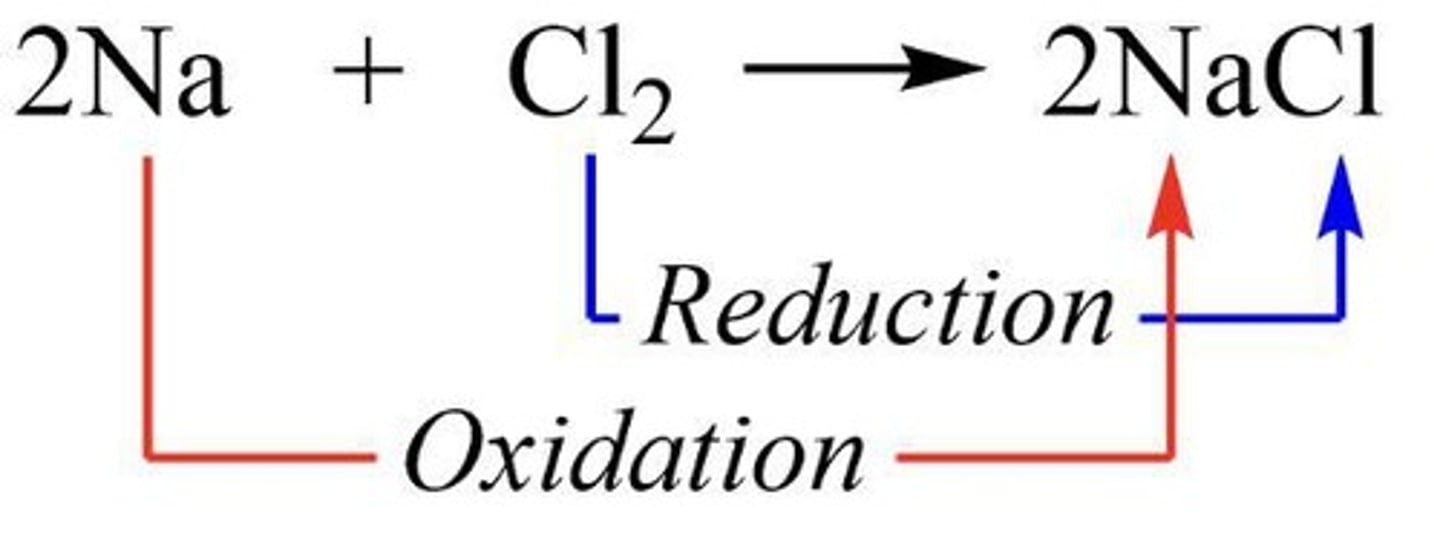
In the reaction Zn + 2H+ → Zn2+ + H2, which element is reduced?
Hydrogen (H) is reduced from +1 to 0.
What is a reducing agent?
The species that donates electrons and is oxidized in the process.
What is an oxidizing agent?
The species that accepts electrons and is reduced in the process.
In the reaction 2Al + 3Cu2+ → 2Al3+ + 3Cu, which species is the reducing agent?
Aluminum (Al) is the reducing agent.
In the reaction 2Al + 3Cu2+ → 2Al3+ + 3Cu, which species is the oxidizing agent?
Copper ions (Cu2+) are the oxidizing agent.
What is the half-reaction for the oxidation of aluminum in the reaction 2Al + 3Cu2+ → 2Al3+ + 3Cu?
Al → Al3+ + 3e-
What is the half-reaction for the reduction of copper in the reaction 2Al + 3Cu2+ → 2Al3+ + 3Cu?
Cu2+ + 2e- → Cu
What oxidation number does iron (Fe) have in Fe3+?
+3
What oxidation number does tin (Sn) have in Sn2+?
+2
In the reaction Fe3+ + Sn2+ → Fe + Sn4+, which species is oxidized?
Tin (Sn) is oxidized from +2 to +4.
In the reaction Fe3+ + Sn2+ → Fe + Sn4+, which species is reduced?
Iron (Fe) is reduced from +3 to 0.