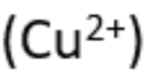Chemistry - Tests
1/19
Earn XP
Description and Tags
Tests for anions, aqueous cations, gases and flame tests for metal ions
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
carbonate
test: add dilute acid
test result: effervescence, carbon dioxide produced
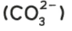
chloride
test: acidify with dilute nitric acid, then add aqueous silver nitrate
test result: white ppt.

bromide
test: acidify with dilute nitric acid, then add aqueous silver nitrate
test result: cream ppt.
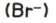
nitrate
test: add aqueous sodium hydroxide, them aluminium foil; warm carefully
test result: ammonia produced

sulfate
test: acidify, then add aqueous barium nitrate
test result: white ppt.
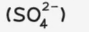
ammonium
effect of aqueous sodium hydroxide: ammonia produced in warming
effect of aqueous ammonia: -
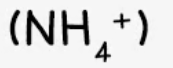
calcium
effect of aqueous sodium hydroxide: white ppt., insoluble in excess
effect of aqueous ammonia: no ppt. or very slightly white ppt.
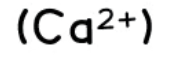
copper (II) - aqueous cation
effect of aqueous sodium hydroxide: light blue ppt., insoluble in excess
effect of aqueous ammonia: light blue ppt., soluble in excess, giving a dark blue solution
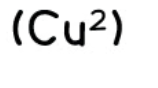
iron (II)
effect of aqueous sodium hydroxide: green ppt., insoluble in excess
effect of aqueous ammonia: green ppt., insoluble in excess
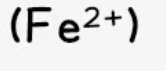
iron (III)
effect of aqueous sodium hydroxide: red-brown ppt., insoluble in excess
effect of aqueous ammonia: red-brown ppt., insoluble in excess
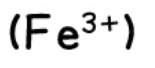
zinc
effect of aqueous sodium hydroxide: white ppt., soluble in excess, giving a colourless solution
effect of aqueous ammonia: white ppt., soluble in excess, giving a colourless solution
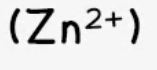
ammonia
test and test result: turns damp red litmus paper blue

carbon dioxide
test and test result: turns limewater milky
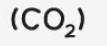
chlorine
test and test result: bleaches damp litmus paper
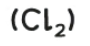
hydrogen
test and test result: ‘pops’ with a lighted splint
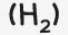
oxygen
relights a glowing splint
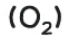
lithium
red flame
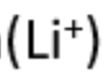
sodium
yellow flame
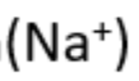
potassium
lilac flame
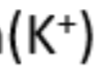
copper (II) - metal ion
blue-green flame