AQUEOUS STOICH
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
Acid/Base Neutralization
Acid + Base → Water + Salt; double replacement
CO2 Formation
Any CO32- or HCO3- + Acid → Solution + CO2 + H2O; double replacement
NH3 Formation
Any NH4+ salt + Strong base → Solution + NH3 + H2O; double replacement
SO2 Formation
Any SO32- salt + acid → Solution + SO2 + H2O
H2CO3 →
CO2 + H2O
NH4OH →
NH3 + H2O
H2SO3 →
SO2 + H2O
Oxidizing Agent
The ion/molecule gaining electrons, allowing for something else to be oxidized
Reducing Agent
The ion/molecule losing electrons, allowing for something else to be reduced.
Electrolysis
Breaks down compound into pure elemental forms. Uses electricity to drive non-spontaneous rxn; type of decomposition
Metal Replacement
metal 1 + metal 2 compound → metal 1 compound + metal 2; only occurs if metal 1 is lower than metal 2 on the SRPC; single replacement
Hydrogen Replacement
only occurs with H2O if metal is below H2O on SRPC; forms H2 + 2OH-. occurs with H+ (actual acid like HCl) if metal is below H+ on SRPC; forms H2
Unreactive Metals
Unreactive metal + OxyAcid → Nonmetallic oxide gas + H2O + salt; only occurs with nitric and sulfuric acid.
metal oxides + non-metallic oxide →
yield a salt;synthesis
metal oxide + water →
yield a metal hydroxide;synthesis
nonmetal oxide + water →
yield an acid;synthesis
metal chlorates →
yield metal chlorides + O2;decomposition
metal carbonates →
yield metal oxide + CO2;decomposition
What makes some solutes electrolytes and some not?
The level of hydration (amount of ions)
Hydration
The process in which an ion or molecule is surrounded by water molecules.
Dissolve
Surrounded by enough water to look like water (clear)
Dissociate
When a compound separates into its ions
Strong electrolytes
Completely dissociate; include soluble ionic salts, the 6 strong acids, and the 8 strong bases
Hydration shell
A structured group of water molecules surrounding a dissolved ion or polar solute, oriented by electrostatic interactions
Strong Acids
HCl, HBr, HI, HNO3, HClO4, H2SO4
Strong Bases
Alkali metal hydroxides, CaBaSr hydroxides
Soluble Ionic Compounds
Alkali metals, NH4+, CH3COO-, HCO3-, ClO3-, NO3-, ClO4-
Usually Soluble (*Insoluble)
[Cl-, Br-, I-]*(MLS)
[F-]*(MLS, CaBaSr, Mg)
[SO42-]*(MLSCaBaSr)
Insoluble Ionic Compounds
metal oxides*(alkali + ammonium), metal hydroxides, phosphates, chromates, dichromates, carbonates, sulfides*(CaBaSr)
Electrolysis
using electricity to drive a nonspontaneous redox reaction
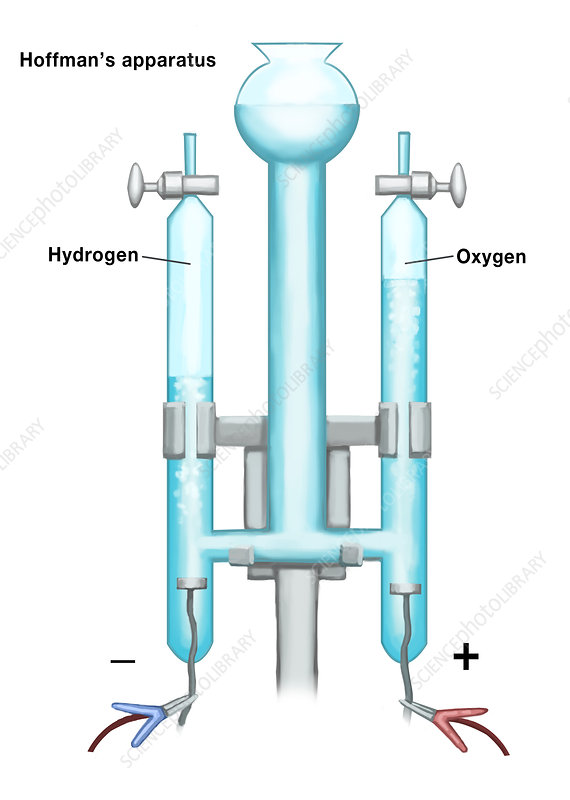
What is the left side called?
Anode
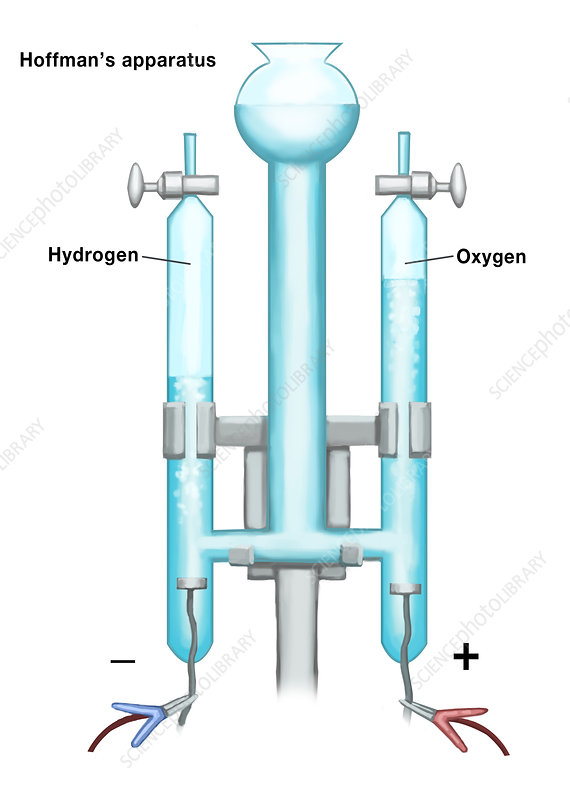
What is the right side called?
Cathode
Anode
electrons leave here in the Hoffman apparatus
Cathode
electrons enter here in the Hoffman apparatus
Bromothymol blue
pH indicator; yellow in acidic conditions <7, blue in basic conditions >7, and green in neutral conditions =7