7-2 Modeling Phase Changes
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
a phase change is when there is ….
transformation between states of matter
during a phase change, energy is spent to ___ instead of ____
break intermolecular forces, increasing particle kinetic energy
vaporization is…
liquid to gas
condensation is…
gas to liquid
sublimation is…
solid to gas
deposition is …
gas to solid
as liquid is heated, particles in liquid gain ____ and ______ energy
vibrational, rotational
when enough energy while heating liquid is gained….
IMFs break
stronger IMFs mean there are…
higher boiling and melting points
are liquids always evaporating?
yes
more kinetic energy =….
more molecules evaporating
temperature is a measure of….
average kinetic energy
boiling happens when….
vapor pressure = atmospheric pressure
all substances boil at a vapor pressure of… (at STP)
101.3 kPa
does NaCl or CsCl have higher melting/boiling point? not imf strength
NaCl
does Al2O3 or NaCl have higher melting/boiling point?
Al2O3
what is one important condition for sublimation to occur at STP?
very weak IMFs
sublimations occurs in solids with _____ that _______
vapor pressures, exceed atmospheric pressure
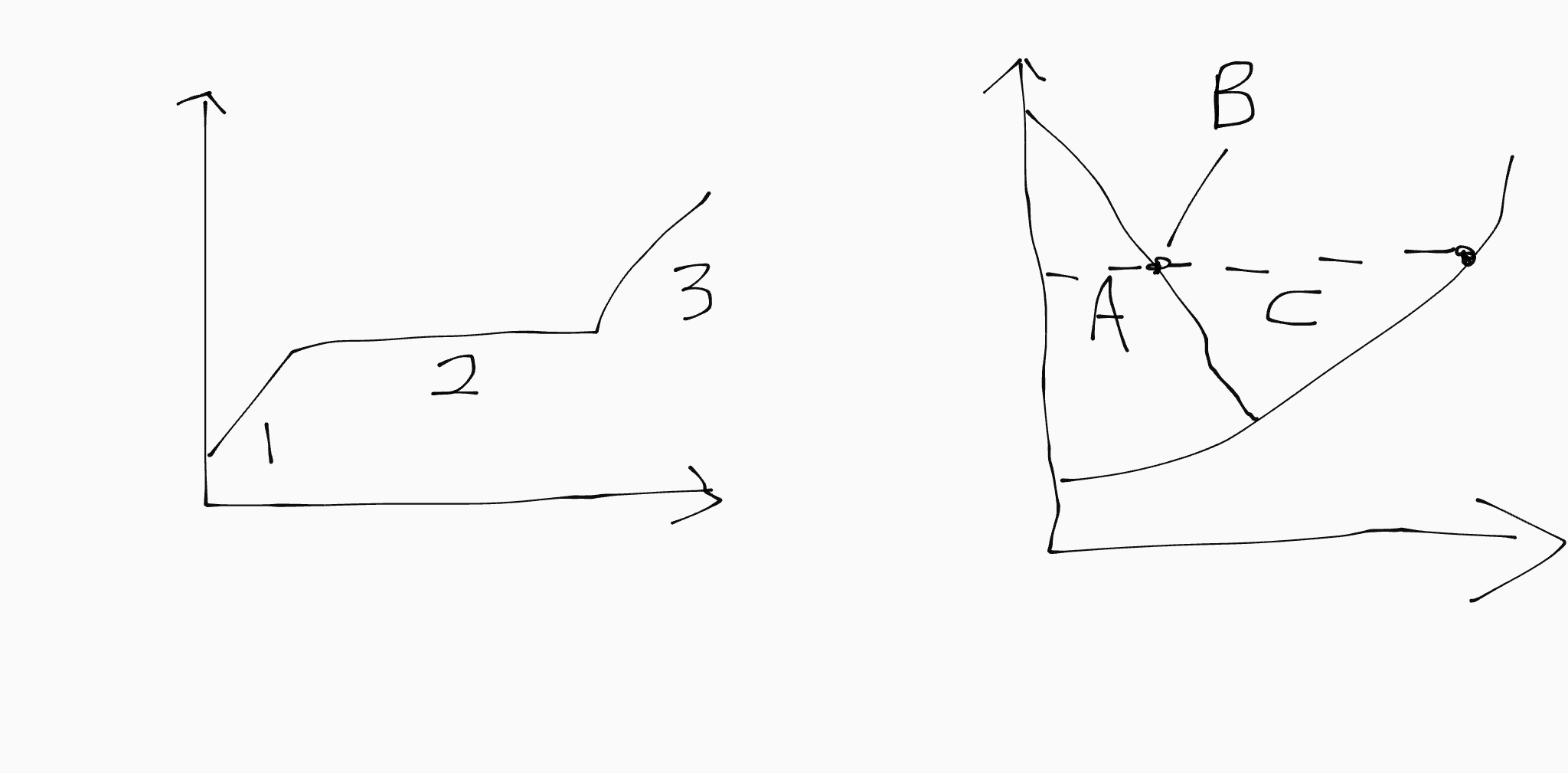
1 corresponds to…
A
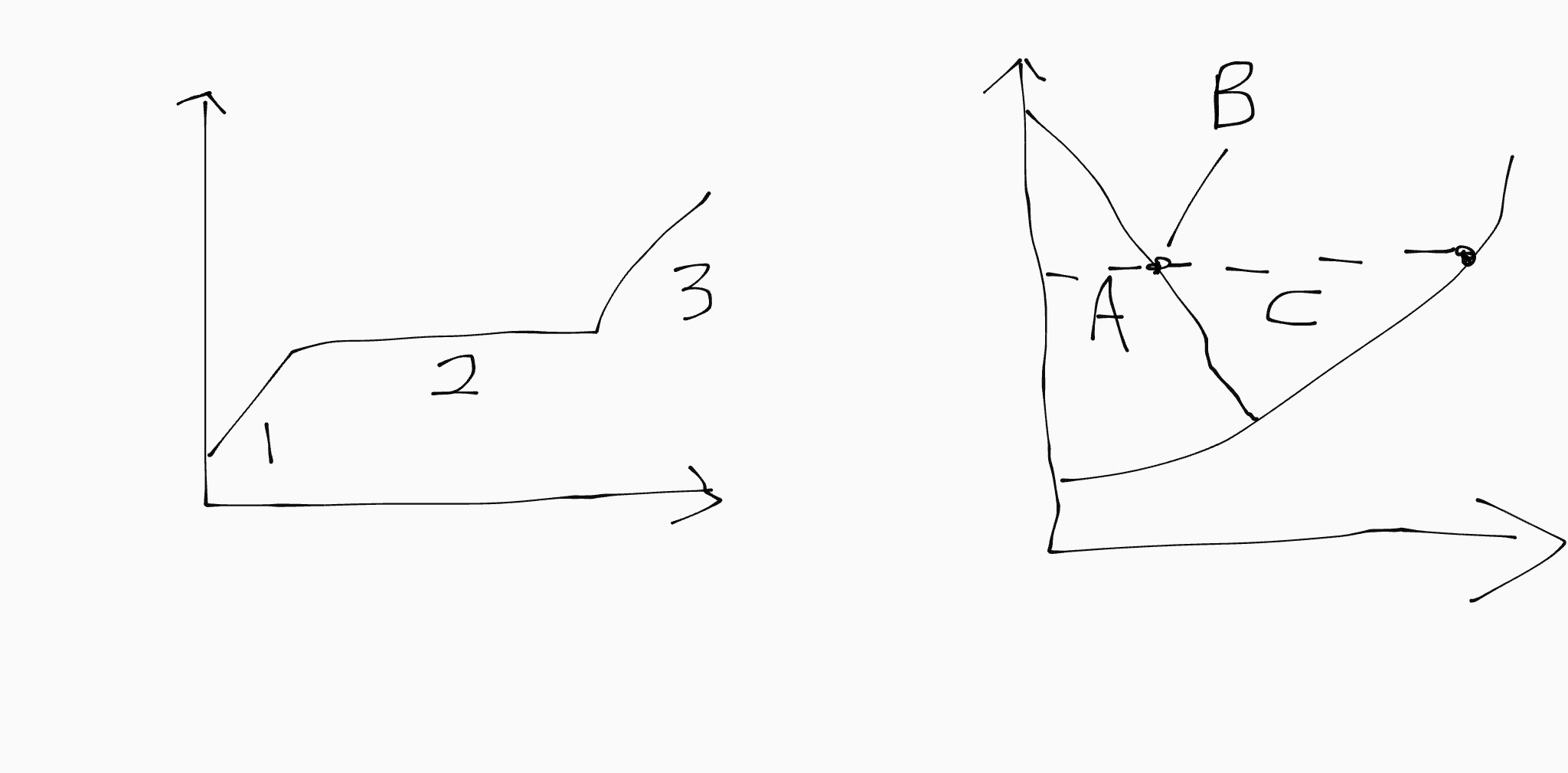
2 corresponds to…
B
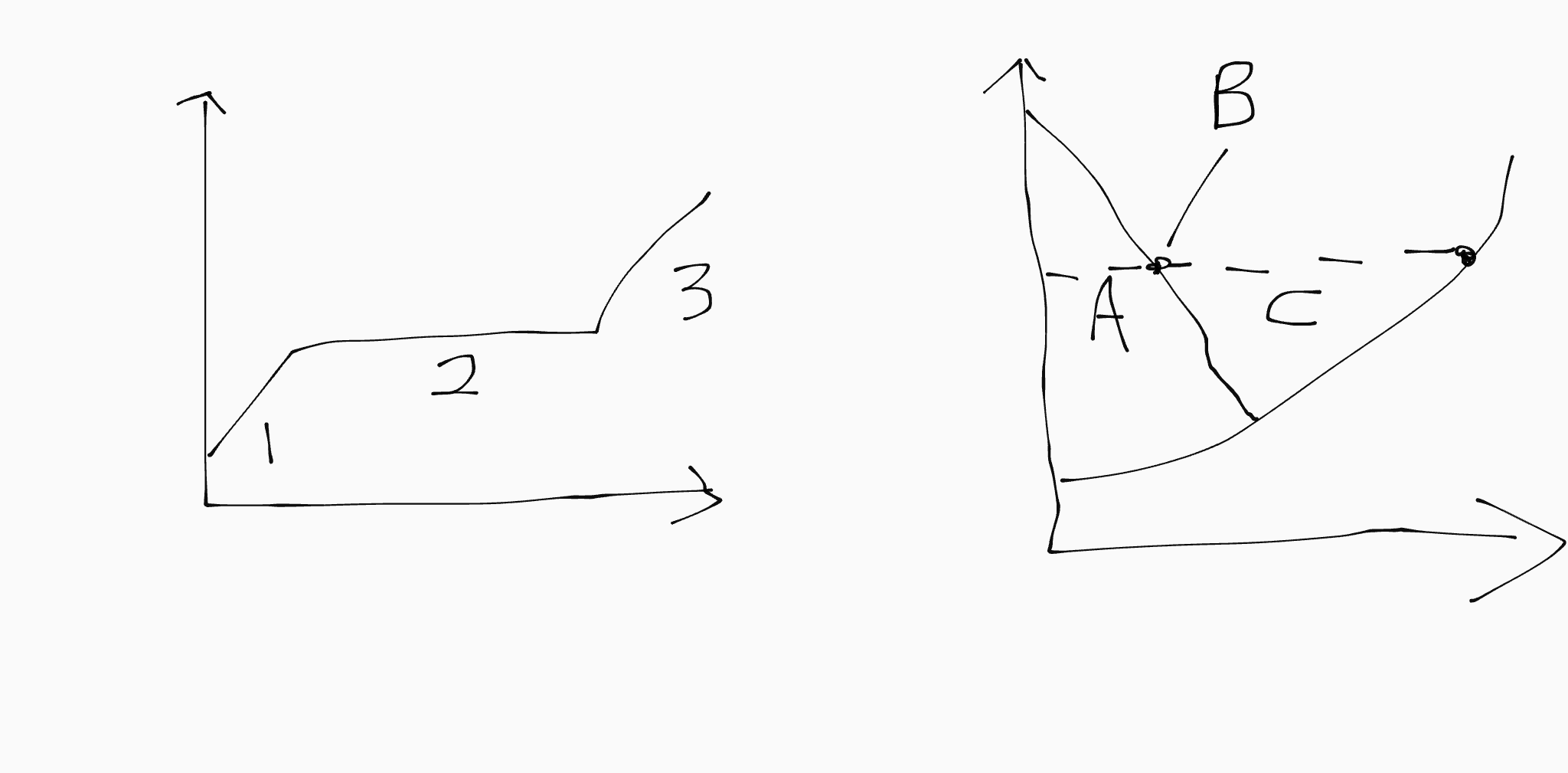
3 corresponds to…
C
what is a critical point?
when liquid and gas coexists