11.2 pressure and volume of ideal gasses relationship
0.0(0)
Card Sorting
1/8
Earn XP
Description and Tags
26/5/25
Last updated 10:47 PM on 5/26/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
1
New cards
P is ______ related to..
inversely related to V
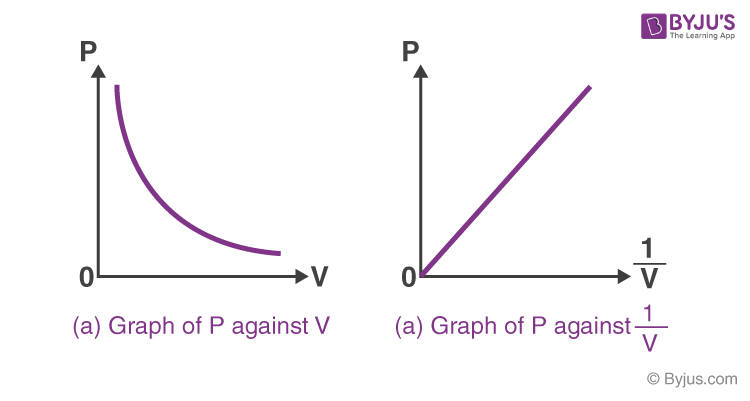
2
New cards
P = k (1/v) or
PV = K
3
New cards
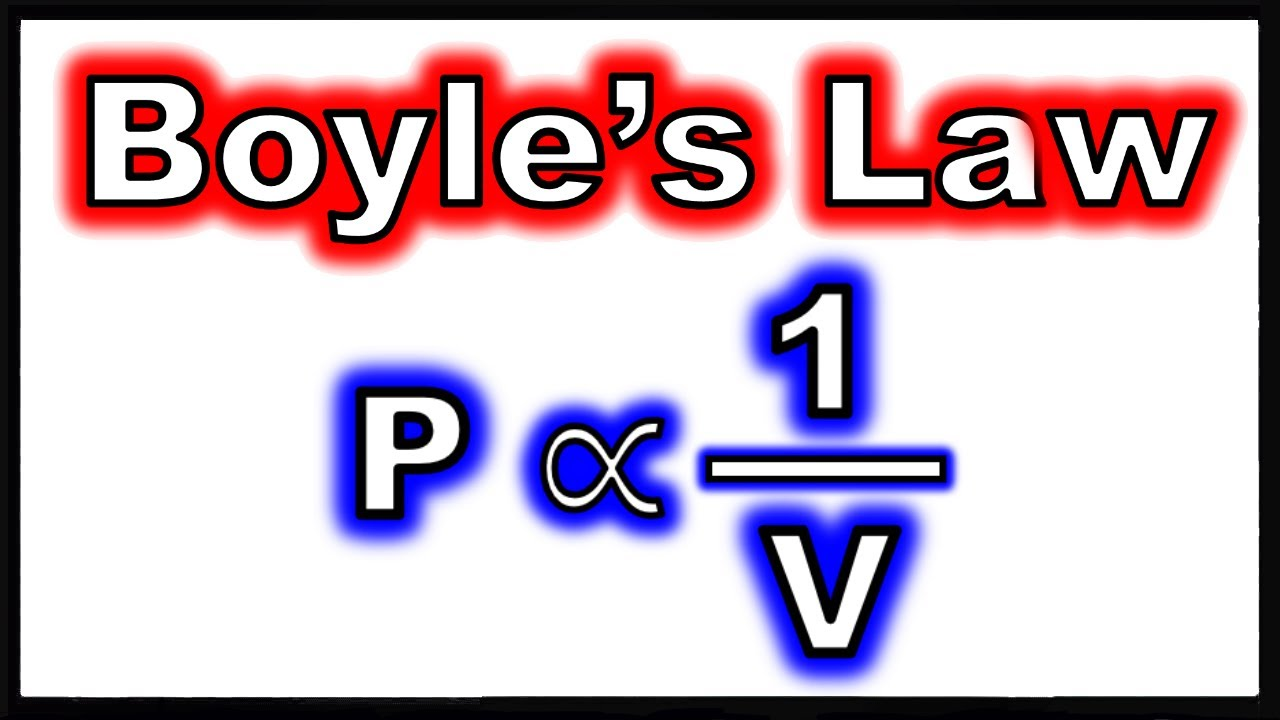
pressure is ________ proportional to
inversely proportional to volume of an ideal gas
4
New cards
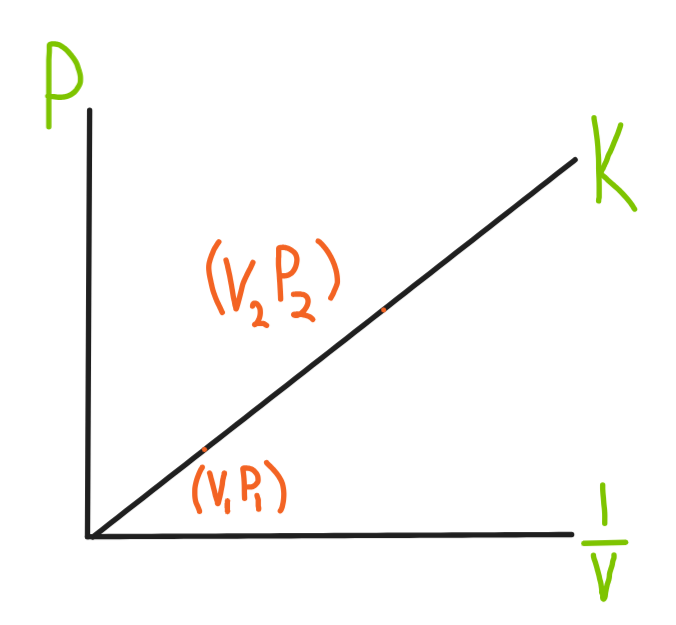
P1 V1 = k, P2 V2 = k
P1 V1 = P2 V2
5
New cards
standard atmospheric pressure: kPa
101.3 kPa
6
New cards
standard atmospheric pressure: atm
1.00 atm
7
New cards
standard atmospheric pressure: mmHg or torr
760.0 mmHg or 760.0 torr
8
New cards
what 3 values are all directly proportional?
101.3 kPa = 1.00 atm = 760.00 mmHg or 760.0 torr
9
New cards
convert 96.8 kPa to mmHg
96.8 kPa/101.3 kPa = x mmHg/760.0 mmHg
x mmHg = 96.8 kPa x 760.0 mmHg /101.3 kPa
= 726.2 mmHg