What I don't know CellBio
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
3 main transport mechanisms
Large pores: cytosol to nucleus (gated transport)
Narrow channels: Cytosol to ER/mitochondria/chloroplast/peroxisome
Vesicles: ER to GA to endosome/lysosome/ PM
Signal Sequence of ER
- hydrophobic
- N terminal with hydrophobic core
- directs transport to ER and is cleaved in ER
functions:
- targeting to ER via binding of SRP
- opening of translocation channel
Different Membrane Proteins
Integral:
- Transmembrane: alpha helix, beta barrel
- Membrane associated
Peripheral membrane protein:
-Lipid linked
-Protein attached
Insertion of Integral Membrane Proteins
1. Insertion of a single-pass membrane protein (2 signals) N-terminus ER signal sequences and transmembrane hydrophobic stop sequence - the ER signal is cleaved-N in lumen and C in cytosol
2. Insertion of single-pass membrane protein (1 signal) - not cleaved as also functions as an anchor sequence - C in lumen
3. Insertion of double pass membrane - anchored by two transmembrane segments
What happens during modification of vesicle Transport?
Glycosylation in lumen of ER- can protect protein and play a role in the folding and sorting
-The precursor oligosaccharide is transferred to Asn in the growing polypeptide chain
- Glycolysation during synthesis is addition of sugars during synthesis of the protein
- Trimming and processing of sugars continues in ER and GA
Quality control
- Proteins that have entered the ER can stay there or move on to the GA
- the default pathway is to move on and there needs to be special mechanisms to stay in the ER
- aggregation or interaction with chaperones prevents packaging
- Retention in the ER via recognition of retention signal in the protein (KDEL or KKXX)
- If ER resident proteins escape to the GA they are recognised by KDEL receptors and shuttled back to the ER
What are the two pathways of exocytosis??
1. Constitutive secretion
- default continuous process
- present in all cells
- permanent flow of materials to membrane
- counteracts endocytosis
2. Regulated secretion
- dependent on stimulus (i.e. hormone)
- present in specialised cells
- storage in vesicles near PM
What are features of lysosomes?
- degradation of macromolecules by hydrolases
- hydrolases have a low pH optimum - this is so that when the lysosome bursts the hydrolases do not work at the cytosol neutral pH
- The membrane of lysosomes:
- protect the cytosol
- contains many transporters - to pump metabolites out
- contains ATPase- to pump proteins in
Explain traffic from GA to lysosomes
1. The patch ss hydrolase is recognised and M6P is attached in the CGN
2. M6P binds to the M6P receptor in TGN
3. Packaging in clathrin coated vesicles
4. Delivery of cargo (hydrolase) at lysosome
5. M6P dissociated due to low pH - can be recycled
6. Phospate is removed from hydrolase and becomes an active mature enzyme
What are the characteristics of intermediate filaments?
- Assemble in a rope-like fashion
-Sturdy
-Has a complementary monomer which twists together
- Anti-parallel binding - hence no polarity as there is a N-terminus at both ends
- 8 tetramers associate
What are the functions of intermediate filaments?
- Distributes the effects of local forces, protecting cells and their membrane from rupture
- They strengthen cells
- Can also form crosslinks with other cytoskeletal networks e.g. with microfilaments
- they are the toughest and most durable
- allow cells to withstand mechanical stress due to stretching
Explain actin tread milling
- monomers are incorporated at the plus end while the minus end is loosing monomers
- this means individual monomers move through the filament which generates movement without changing the length of the filament
- actin tread milling drives actin-driven motility
How do actin filaments mediate directed movement of cells
- tread milling is critical
- moves the cell
-migrates based on specific cues
- receptors sense the environment
-actin polymerisation occurs at the plus end and the lamellipodium potrudes / the plasma membrane protrudes and forms new actin cortex
- new points of anchorage are made between the bottom of the cell and the surface
- contraction at the rear end of the cell are mediated by myosin motor proteins moving along the actin filament which draws the body of the cell forwards
- new anchorage points are established at the front and old ones are released at the rear
What is the function of Arp (actin-related protein) complex??
Nucleates actin filaments from the sides of existing filaments
- this branching is always at 70 degrees
- this branching gives rise to filopodia
How is the cell cycle initiated?
- Activation of G1-Cdk by mitogens and the MAP kinase pathway
- mitogen is an extracellular signal from another cell
- mitogens act by switching on cell signalling pathways that stimulate the synthesis of G1 cyclins and G1/S cyclins
1. Mitogen activates RAS which activates MAP kinase
2. This activates the gene regulatory protein
3. There is immediate gene expression and a gene regulatory protein is formed
4. There is delayed-response gene expression of the cyclin
What is the function of APC/C?
APC/C is a protein that adds ubiquitins
- it activates separase which breaks down cohesins
-it also breaks down all the cyclins
- it is activated by M-CDK
How is cytokinesis triggered?
By the dephosphorylation of Cdks and cyclin breakdown by APC/C ubiquitination
How does DNA damage block cell division?
- DNA damage causes phosphorylation of p53
- p53 binds to p21 gene
- p21 translation produces a protein that inhibits Cdk
- mutations in p53 are a risk factor for cancer as this causes uncontrolled cell division
What are hallmarks of apoptosis?
Extracellular:
- cell looses its shape and shrinks
- the plasma membrane forms protrusions
- cell debris is recognised by macrophages that clear this
Intracellular:
-the nuclear envelope disintegrates and chromatin is degraded
- degradative vacuoles are formed
- disassembly of the cytoskeleton
- degradation of mitochondria and release of cytochrome
How is the caspase cascade activated in apoptoses?
Procaspases are inactive caspase precursors that become active when cleaved into large and small subunits, discarding the prodomain. One active initiator caspase then activates many executioner caspases, which cleave cytosolic proteins and nuclear lamins to drive apoptosis.
Extrinsic apoptosis
Extrinsic apoptosis is triggered when a killer lymphocyte’s Fas ligand binds Fas death receptors on a target cell, forming the DISC that activates caspase‑8, which then turns on executioner caspases to kill the cell.
Intrinsic (mitochondrial) pathway
Intrinsic (mitochondrial) pathway: Bcl‑2 on mitochondria normally blocks cytochrome c release; apoptotic signals activate BH3‑only proteins, which inhibit Bcl‑2 so Bax/Bak form pores, cytochrome c is released, and caspases are activated.
Survival signaling blocks apoptosis
Increasing transcription of anti-apoptotic Bcl-2 proteins
Akt-mediated phosphorylation and inactivation of BH3-only proteins (e.g. Bad)
MAP kinase–mediated inactivation of IAP antagonists (e.g. Hid), allowing IAPs to inhibit caspases
Net result: cytochrome c release prevented, caspases inhibited, apoptosis blocked.
What is the structure of GPCRs?
- all have 7 transmembrane helices
- all bind heterotrimeric G-protein complex consisting of three subunits; alpha, beta and gamma
- the G-protein complex traduces the signal inside the cell
What are the four major classes of Ga subunits that differentially control downstream signalling?
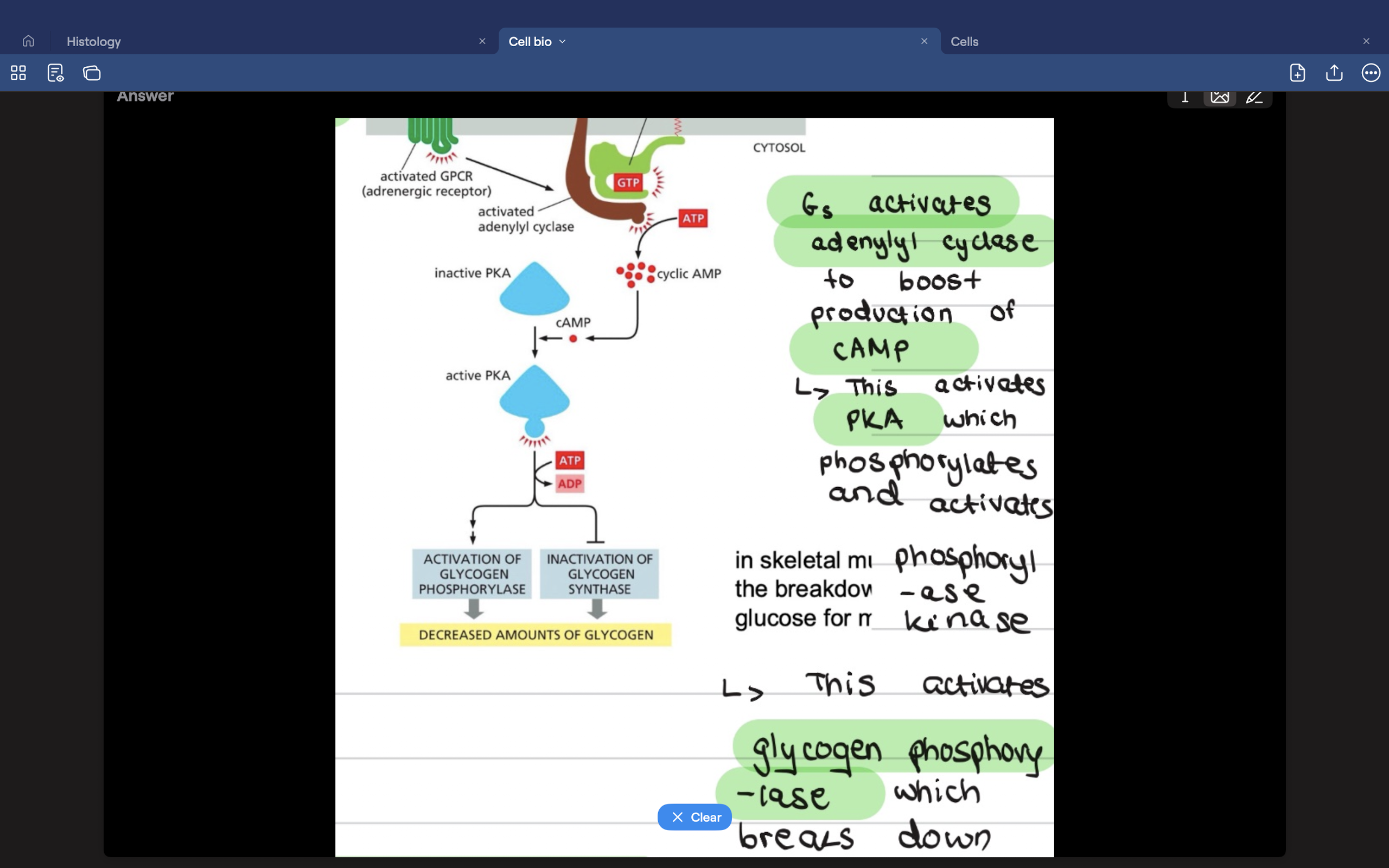
Gas- stimulates adenylyl cyclase activity- increase cAMP
Gai- inhibits adenylyl cyclase activity - decreases cAMP
Gaq - activates phospholipase c signaling
Ga 12/13 - activates small GTPases that act on the actin cytoskeleton
What is the Gai/s pathway
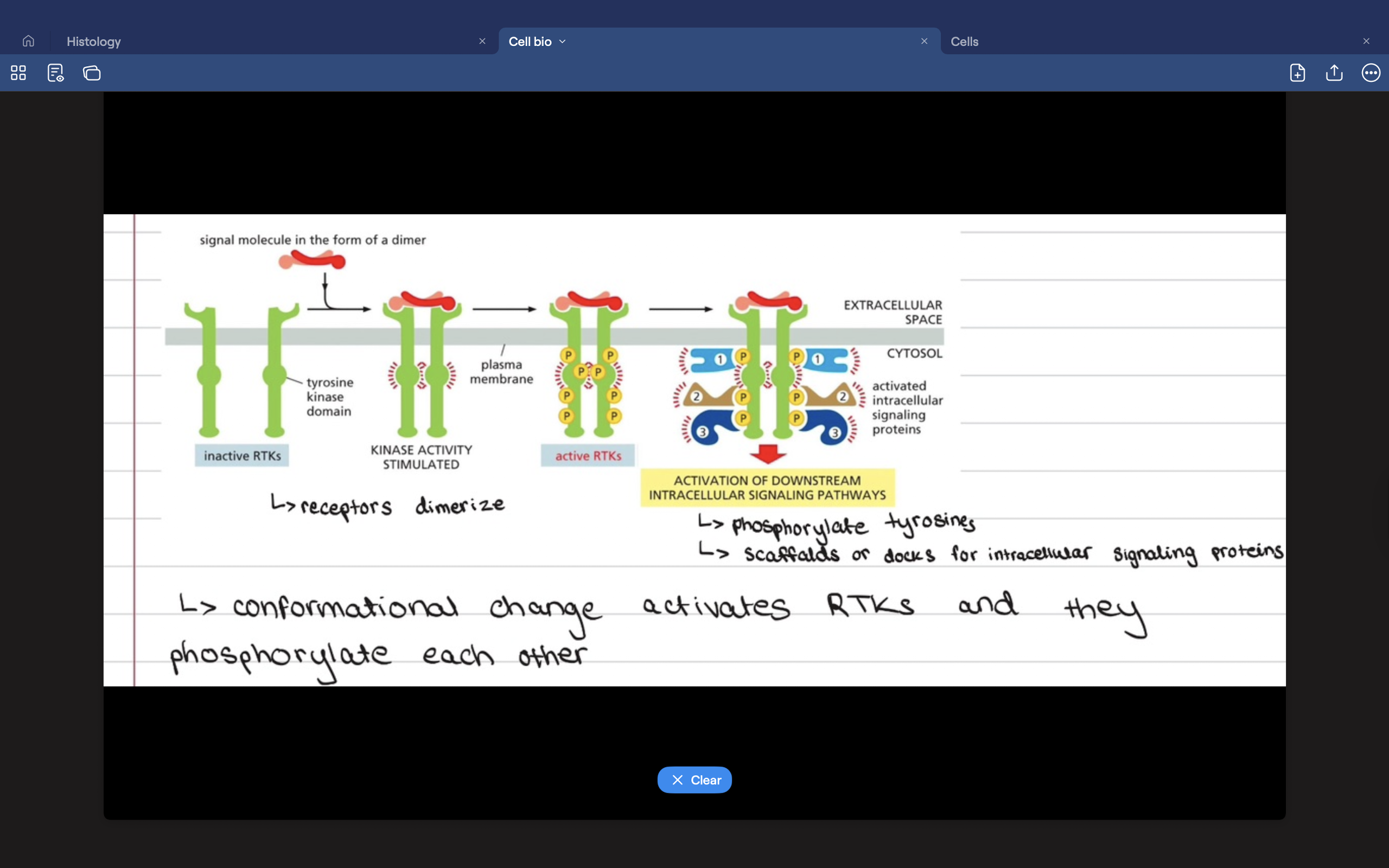
What are receptor tyrosine kinases?
The most common enzyme-coupled receptors.
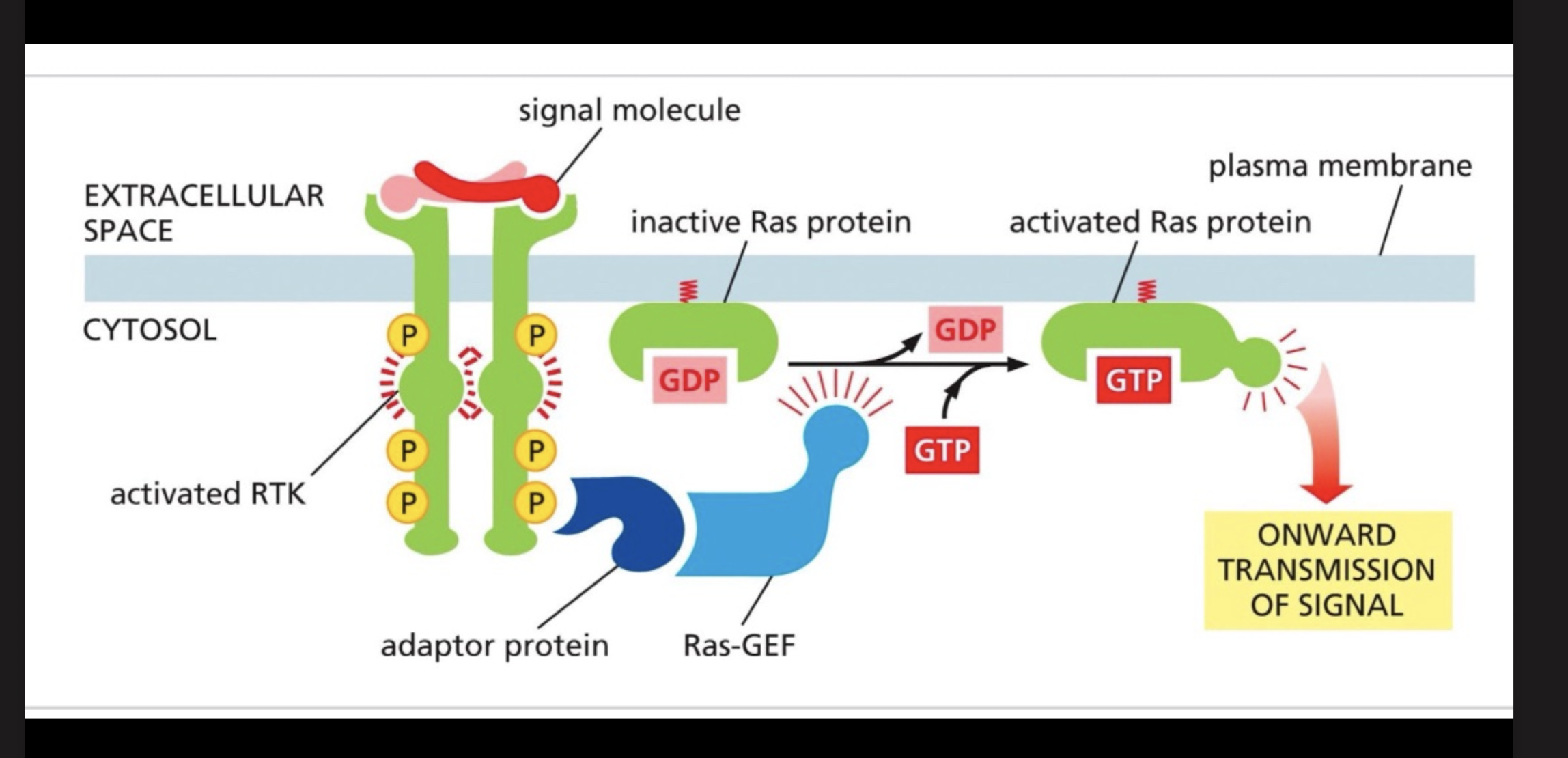
How does tyrosine kinase activate the RAS pathway?
Mainly also activates the MAP pathway.
Why is the ideal signal transduction curve s-shaped?
This means that there is a smaller uncertainty and there is a threshold for when a signal just be transduced.
What is ultra sensitivity?
Output is extremely sensitive to input in threshold
What is cooperativity?
e.g. hemoglobin - the binding at one site influences binding at another site
What is zero-order ultra sensitivity?
- a small change in vmax (vmax of kinase gets below that of the PP) causes a large shift in non phosphorylated protein (a lot more)
- called zero order because it is independent of the concentration
- km must be very low
What prevents self-assembly of collagen in the fibroblast?
Collagens are produced and secreted as procollagens and then pro collagen extensions are cleaved by proteinases in the EM
Integrins
Connects the intracellular cytoskeleton to Extracellular Matrix. Through Fibronectin.
Active via binding to fibronectin or to cytoskeleton
- binding unfolds interns
- activity can be modulated by intracellular signals
- via this mechanism a fibroblast can attach to and release from a substrate
- kinases and phosphates regulate binding