Physics II Exam 3 Vocabulary
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
What is a photon?
smallest possible excitation of the electromagnetic field at a given frequency
What is an electron?
negatively charged, subatomic particle and a fundamental component of all atoms
What is a neutron?
subatomic particle that has no electric charge, slightly greater mass than a proton
What is a proton?
subatomic particle with a positive electric charge
What is the function of a photon?
changes energy state (excitation) of atom
What is the function of an electron?
changes the charge of an atom to produce an ion
What is the function of a neutron?
changes the mass of an atom to make a different isotope
What is the function of a proton?
change of atomic species, transmutation
What is the product of alpha decay?
helium nucleus
What is the product of beta decay?
electron
What is the product of gamma decay?
photon
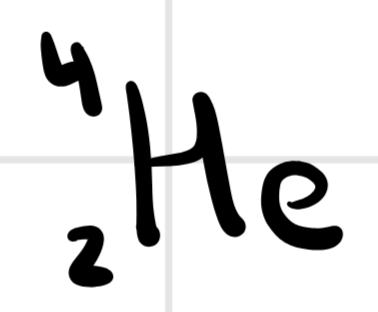
What does this notation represent?
alpha decay
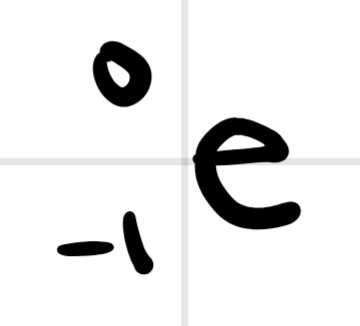
What does this notation represent?
beta decay
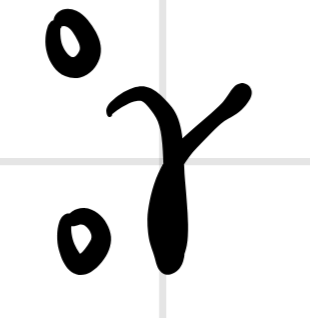
What does this notation represent?
gamma decay
What is the unit used to measure the activity of radioactivity?
curie Ci
What does a curie (Ci) represent?
fixed number of decays per second
What is the definition of half-life?
the time needed for half of the active nuclei in a sample to decay
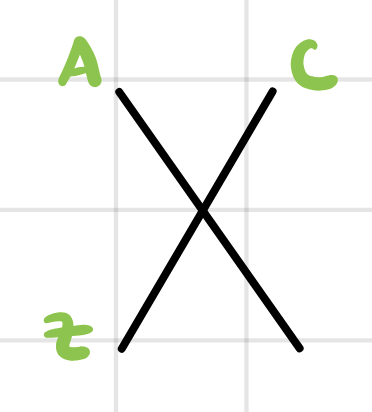
What does Z represent?
atomic number which is the number of protons which gives the species of the atom
What does A represent?
mass number which is the sum of the protons and neutrons together that determines the isotope of the atom
What does C represent?
charge number which gives the charge (ion) status of the atom