quantum phenomena
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
describe the photoelectric effect
when UV light is shone on a metal, electrons are emitted
condition for photoelectric effect
frequency of light must exceed threshold frequency
is there a time-lag for the photoelectric effect?
no, electrons are immediately emitted
effect of increasing intensity on the photoelectric effect
more photoelectrons are emitted as increased intensity means there are more photons
effect of increasing frequency on the photoelectric effect
higher max KE
define threshold frequency
minimum frequency of photons required for photoelectrons to be emitted from the surface of a metal plate through the photoelectric effect
equal to the metal’s work function divided by Planck’s constant
define the work function of a metal
minimum energy required to remove an electron from a metal’s surface
define stopping potential
minimum potential difference required to stop the highest kinetic energy electrons from leaving the metal plate in the photoelectric effect
what can we do after measuring stopping potential
use it to find the max KE of the released photoelectrons
max KE = eVs
describe excitation
electrons in atoms can only exist in discrete energy levels
these electrons can gain energy from collisions with free electrons
this causes them to move up an energy level
describe ionisation
when the energy of the free electrons is more than the ionisation energy, electrons gain enough energy to be removed from the atom entirely
define excitation
the process of an electron taking in exactly the right quantity of energy to move to a higher energy level
define ionisation
the process of an atom losing an orbital electron and becoming charged
what happens when an electron becomes excited
it quickly returns to its original energy level (ground state) and releases the energy it gained, in the form of a photon
use of excitation
fluorescent tubes
explain how fluorescent tubes work
filled with mercury vapour, across which a high voltage is applied
this voltage accelerates free electrons through the tube and they collide with mercury atoms and become ionised so release more free electrons
free electrons collide with mercury atoms and become excited
when they de-excite they release photons, mostly within the UV range
fluorescent coating on the inside of the tube absorbs the photons
therefore the electrons in the atoms of the coating become excited and de-excite, releasing photons of visible light
define electron volt
energy gained by (work done on) 1 electron when passing through a potential difference of 1V
1 eV =
1.6 × 10^-19 J
wave property of electrons
electron diffraction
De Broglie
if light has particle properties, particles also have wave properties
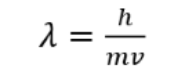
effect of increased momentum on diffraction
wavelength decreases
diffraction decreases
concentric rings of the interference pattern are closer together
Explain how line spectra are produced.
In your answer you should describe:
• how the collisions of charged particles with gas atoms can cause the atoms to emit photons.
• how spectral lines are explained by the concept of discrete energy levels.
• Energy from collision of charged particles transfers to electrons in gas molecules.
• Electrons excited to higher energy levels
• The more energy the electrons absorb the higher the energy levels reached.
• Electrons are unstable at higher energy levels so will fall back down.
• When it falls down it will emit a photon
Formation of spectral lines:
• Photon energy = hf/ or photon energy proportional to frequency.
• Spectral lines are at specific wavelengths.
• Each spectral line corresponds to an electron falling down to a lower energy state.
• Energy gap, ΔE = hc/λ
• Larger energy gap means higher energy photon is emitted so shorter wavelength or vice versa.