Reactivity series + iron/rust
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
What is the order of reactivity? Include positions of carbon and hydrogen
Potassium, sodium, lithium, calcium, magnesium, aluminium, (carbon), zinc, iron, (hydrogen), copper, silver, gold
Which ones can react with both water and dilute (hydrochloric or sulfuric) acid, only acid or don't react with dilute acid?
Both: potassium, sodium, lithium, calcium
Only acid: magnesium, aluminium, zinc, iron
Do not react with dilute acid: copper, silver, gold
How does Mg, Al, Zn and Fe react?
Mg vigorously, Al slow (Al oxidises quickly so a layer of aluminium oxide forms a coat around it. Once it is gone it starts reacting slow with acid), Zn and Fe need the acid to be warmed up.
Wha is the word equation for the reactions between metals and water?
Metal + water ➝ metal hydroxide + hydrogen
Write both the word and chemical equation for these reactions: calcium and water, lithium and water
Calcium + water ➝ calcium hydroxide + hydrogen
Ca(s) + 2H2O(l) ➝ Ca(OH)2 (aq) + H2 (g)
Ca ions have a +2 charge/ OH ions have a 1- charge
Lithium + water ➝ lithium hydroxide + hydrogen
2Li(s) + 2H2O(l) ➝ 2LiOH(aq) + H2(g)
What is the word equation for the reaction between metals and an acid?
Metal + acid ➝ metal salt + hydrogen
What will happen to a strong acid when dissolved in water?
It will dissociate, meaning that the hydrogen ions are separated from the molecule.
The more reactive an acid the more H+ ions are released into solution (do not confuse with how many hydrogen atoms it has — HCL is a stronger acid than H3PO4 even though it has less H atoms but it will release more H+ ions quickly because it allows complete dissociation whereas H3PO4 only partially dissociates so more hydrogen atoms but less H+ ions).
Remember that a dilute acid is a stronger than concentrated acid IF IN AQUEOUS SOLUTION because it allows complete dissociation of the acid
What are the chemical formulas of these acids? What salts with they produce? How do we know the charges?
Hydrochloric acid, sulfuric acid, nitric acid, phosphoric acid
Hydrochloric acid (HCl) produces chloride salts
Sulfuric acid (H2SO4) produces sulfate salts
Nitric acid (HNO3) produces nitrate salts
Phosphoric acid (H3PO4) produces phosphate salts
Some of them we had to learn but if not sure look at how many hydrogen atoms there are. Hydrogen has a +1 charge so if there are 3 hydrogen molecules the charge of the other compound will be 3-.
E.g = phosphoric acid has 3 hydrogen molecules so the charge of PO4 is equal to 3-
Give me the word and chemical equations for:
Potassium and nitric acid.
Zinc and phosphoric acid.
Copper annd sulphuric acid.
Potassium + nitric acid ➝ potassium nitrate + hydrogen
2K(s) + 2HNO3(aq) ➝ KNO3(aq) + H2(g)
Zinc + phosphoric acid ➝ zinc phosphate + hydrogen
3Zn(s) + 2H3PO4(aq) ➝ Zn3(PO4)2(aq) + 3H2(g)
Copper + sulphuric acid ➝ No reaction
Practical: investigation into reactions between metals and dilute acids
Method?
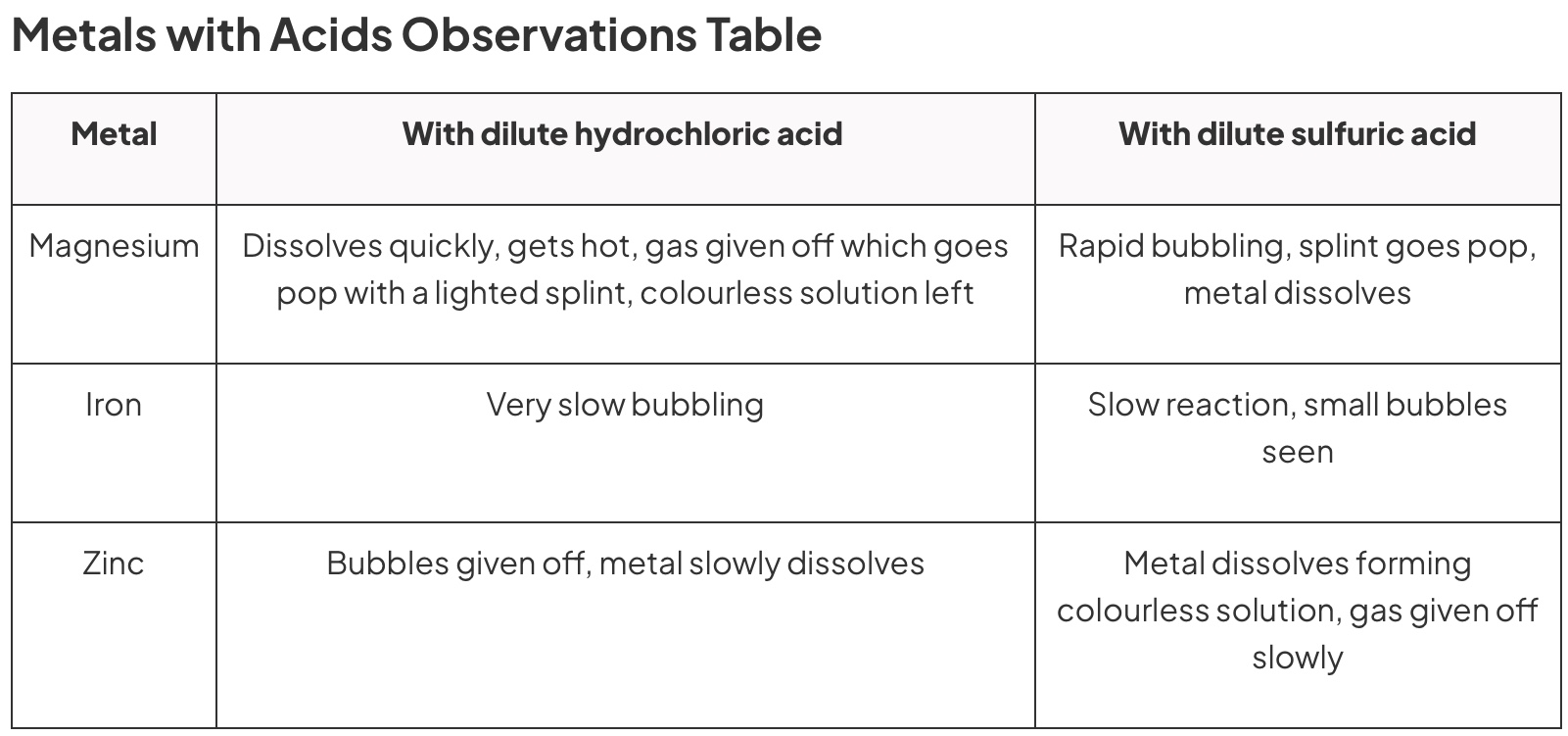
Table of observations of reaction between dilute hydrochloric acid and magnesium, zinc, iron and copper

Safety equipment? Why? + other dangers
Wear eye protection: some acid droplets might be carried out by the gas
Calcium can cause irritation when it reacts with water in skin
Magnesium is flammable
These reeaction are all ____
How can we use this to compare reactivity of these metals? To make a valid test what control variables we must control?
Exothermic
We can measure the temperature change.
Control variables:
Volume and concentration of hydrochloric acid
Moles of metal
Particle size/surface area of metal
How does measuring the temperature change tell us about the reactivity? How does it work, not just smaller temp change = less reactive
How does surface area/particle size affect it?
With a less reactive metal the reaction occurs very slowly, so the heat will be given out over a long period of time. As the heat is being given out, it will also be lost to the surroundings and the temperature change will be quite low.
If the reaction occurs more quickly, the heat is given out more quickly to the solution and there is less time for heat to be lost to the surroundings so we obtain a larger temperature change.
Surface area affects the speed at which the heat is given out. A large surface area causes a faster rate of reaction, affecting temp change. We should use metal powders in these reactions
Why do we use a polystyrene cup? What safety measures should we follow and why?
Insulator so the least heat escapes
Wear eye protection and avoid splashing the acid when stirring
After finding the data (temperature change) for each metal, in what type of format should we put it?
A bar chart because the type of metal is not continuous data: the metal can be either one or the other, it can’t be anything in between
There is nothing between magnesium of zinc, it can only be either magnesium or zinc
Remember displacement reactions. Look carefully at the reactivity series to see if any metals will displace other metals. Write the chemical formulae fo these reactions:
Magnesium + copper sulphate
Zinc + copper nitrate
Aluminium + iron(II) sulphate
Mg(s) + CuSO4 (aq) ➝ MgSO4 (aq) + Cu (s)
Zn (s) + Cu(NO3)2 (aq) ➝ Zn(NO3)2 (aq) + Cu (s)
2Al (s) + 3FeSO4 (aq) ➝ Al2(SO4)3 (aq) + 3Fe (s)
Complete the table with the observations:
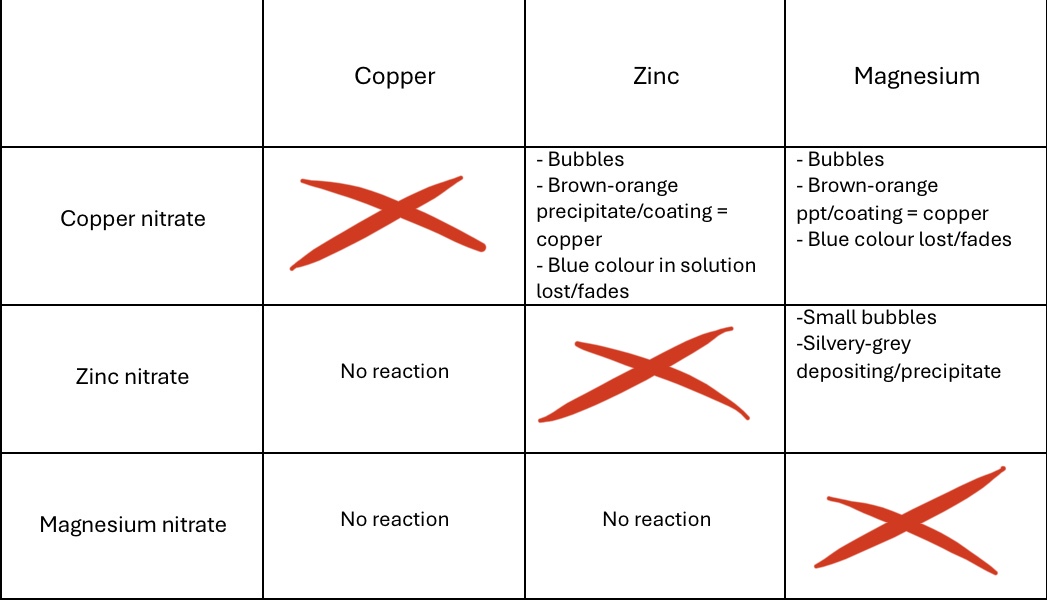
In displacement reactions:
What is formed when we see a brown-orange ppt? What is the outer coating on a block (not powder, powder forms ppt) of zinc (usually zinc)?
Both are copper
How can we identify the order of reactivity with unknown metals?
By reacting them with acid and water and see the rate of reaction (which one is more vigorous)
If two of them don't react with anything then use displacement reaction with one substance's oxide or the aqueous solution of its metal salt and see if any reaction occurs.
A metal will displace another metal from its oxide that is lower in the reactivity series
A metal will also displace another metal from its salt that is lower in the reactivity series
INFO on hydrated copper sulfate
Formulas for hydrated copper sulfate + colours
CuSO4 (s) + 5H2O (l) → CuSO4•5H2O (s)
Anhydrous (=no water) copper sulfate + water → hydrated copper sulfate
Anhydrous copper sulfate is white
Hydrated copper sulfate is blue
Formulas for rust (remember that in IGCSE rust is ONLY used when talking about iron). How can we speed up the reaction of rusting?
Iron + water + oxygen → hydrated iron(III) oxide
4Fe (s) + 2𝑥H2O + 3O2 (g) → 2Fe2O3•𝑥H2O
𝑥 is replaced by a number. In 2𝑥H2O we would multiply 𝑥 by 2.
We can speed up the reaction with sodium chloride
Why do we use iron to build things? Why is rust bad for building?
We use iron because it is strong and malleable.
However, rust is flaky and weak and the material may not move properly.
What are the methods to prevent rusting?
Barrier method
Galvanising
Sacrificial protection
How does the barrier method work? What is a downside of this?
Prevents the iron from coming into contact with water and oxygen by:
Coating the iron with paint
Coating it in oil or grease
Coating it with plastic
If coating is damaged (scratched, washed away), iron is exposed again
How does the galvanising method work? Why is it more effective than normal barrier method?
Coating completely the iron with zinc.
Prevents water and oxygen from getting to the iron and ZnCO3 is formed when zinc reacts with oxygen.
It is more effective because even if the barrier is broken, zinc is more reactive so it will corrode before iron. During the process zinc loses electrons to form zinc ions.
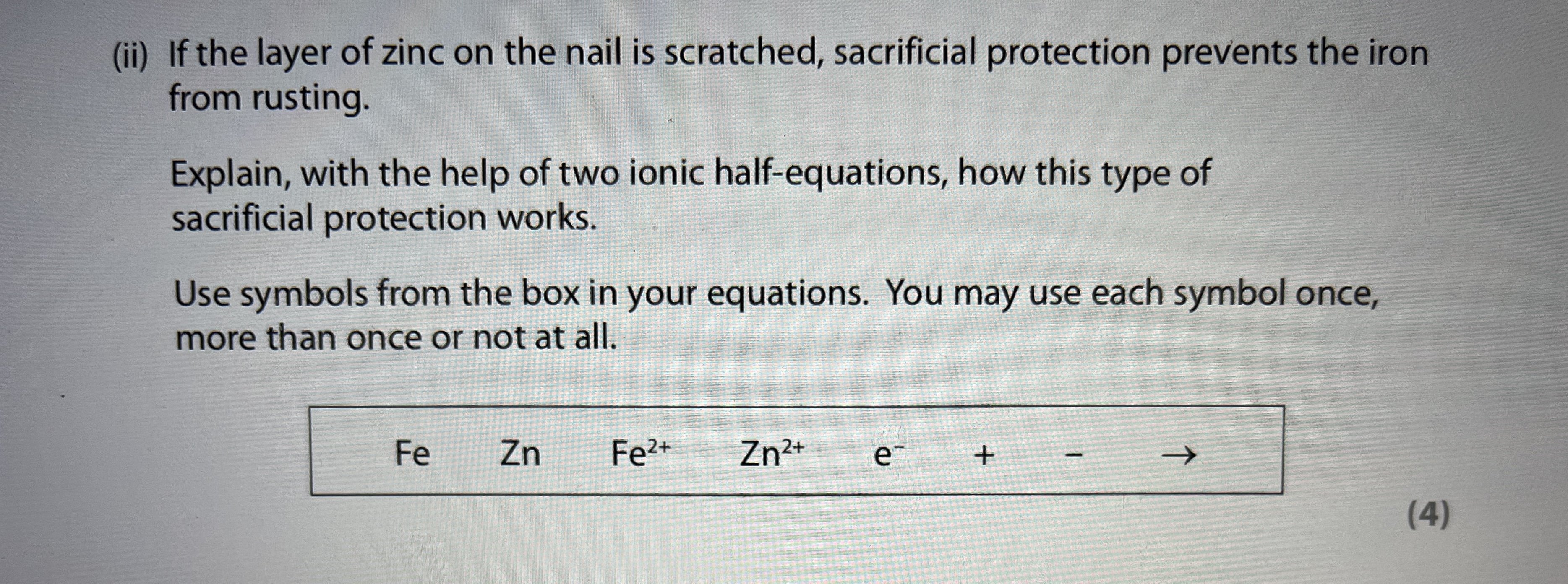
How does the sacrificial protection work?
Blocks of zinc, aluminium or magnesium are attached to the iron structure to act as sacrificial anodes. These metals are more reactive than iron so they will corrode before/instead of iron rusts forming the metal oxide.
Replaced occasionally when all the more reactive metal has been oxidised. Used in large structures because difficult to use barrier method effectively
NOTE: Even though these metals are more expensive than iron, in the long run it will be cheaper. There are sufficient blocks so that they attract the negative electrons.