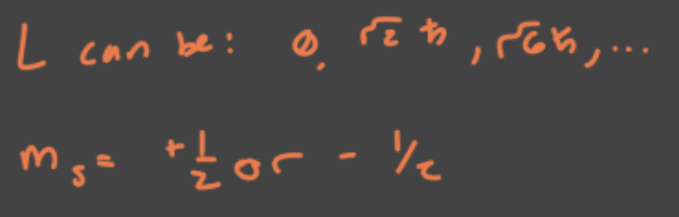Pchem 2 Chapter 7 The Hydrogen Atom
0.0(0)
0.0(0)
Card Sorting
1/10
Earn XP
Description and Tags
344, 345, 346
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
1
New cards
Square of total angular momentum
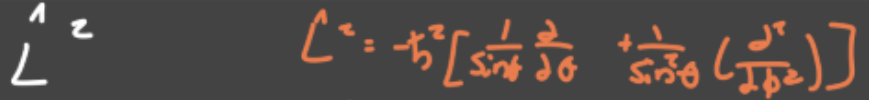
2
New cards
two parts
Stern and Gerlach found the quite unexpected result that a beam of silver atoms splits into only ____. Note that this corresponds to 2l + 1 = 2, or to l = 1/ 2.
3
New cards
doublet
under high resolution it was observed that the n = 2 to n = 1 transition in atomic hydrogen is split into two closely spaced lines, called a ____.
4
New cards
two distinct states
Wolfgang Pauli showed that all these observations could be explained with the postulate that an electron can exist in ____.
5
New cards
spin quantum number m(s)
Fourth quantum number. Restricted to two values, +1/2 and -1/2.
6
New cards
angular momentum eigenvalue equation
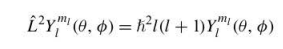
7
New cards
orbital angular momentum of an electron in a hydrogen atom
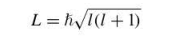
8
New cards
spin angular momentum of an electron in a hydrogen atom
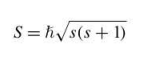
9
New cards
spin eigenfunctions
alpha and beta
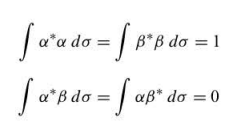
10
New cards
spin variable
sigma, not a continuous variable
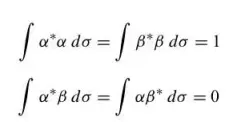
11
New cards
momentums