Chemistry unit 2.4 chemical reactions and energy
1/14
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What is an exothermic reaction?
A reaction where the energy transfers to surroundings so temperature of surrounding increases
What is an endothermic reaction?
A reaction where the energy is taken in from surroundings so temperature of surroundings decreases
What are examples of exothermic reactions
Combustion, neutralisations and oxidation
What are examples of endothermic reactions
Thermal decomposition and electrolysis
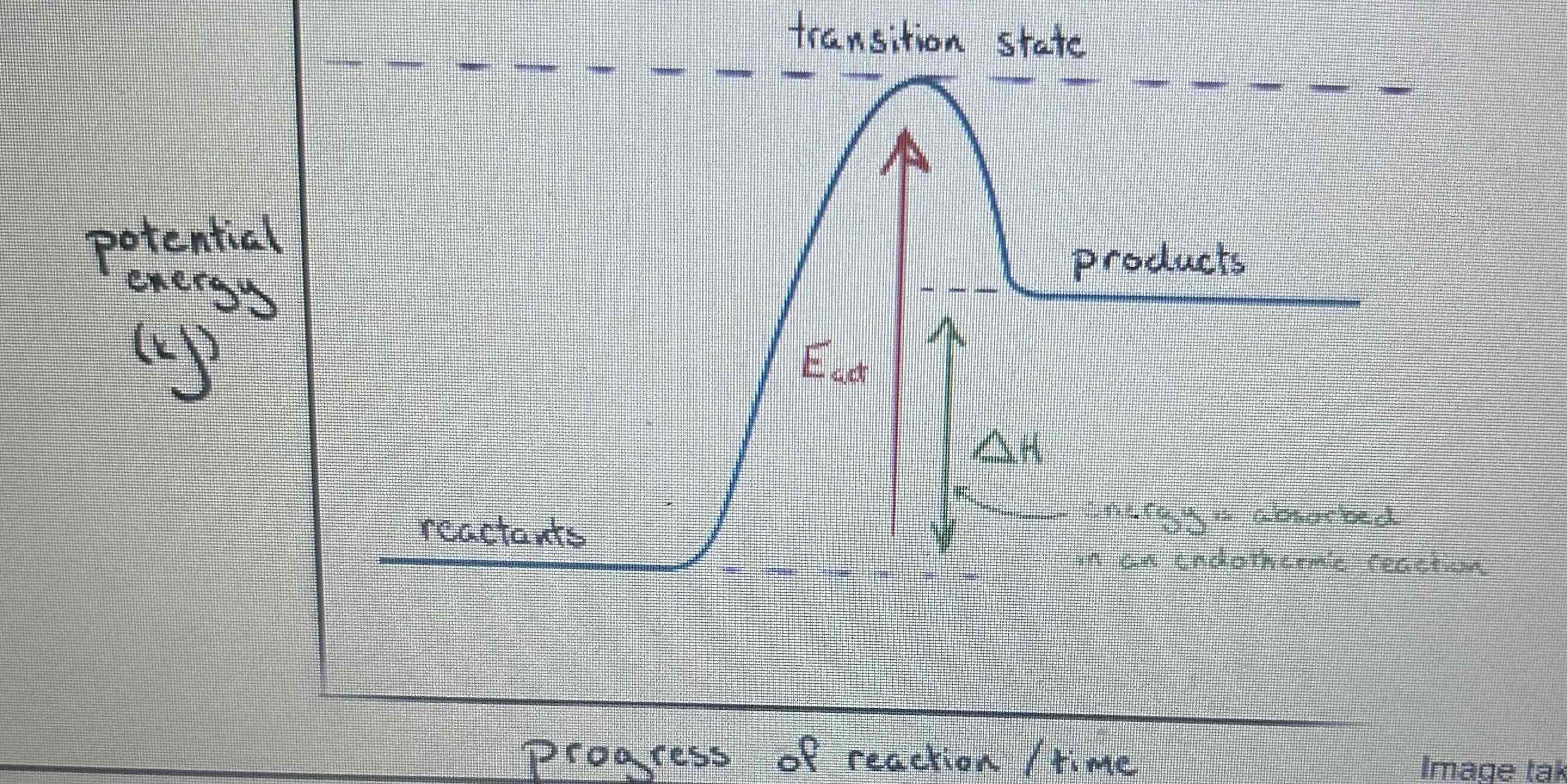
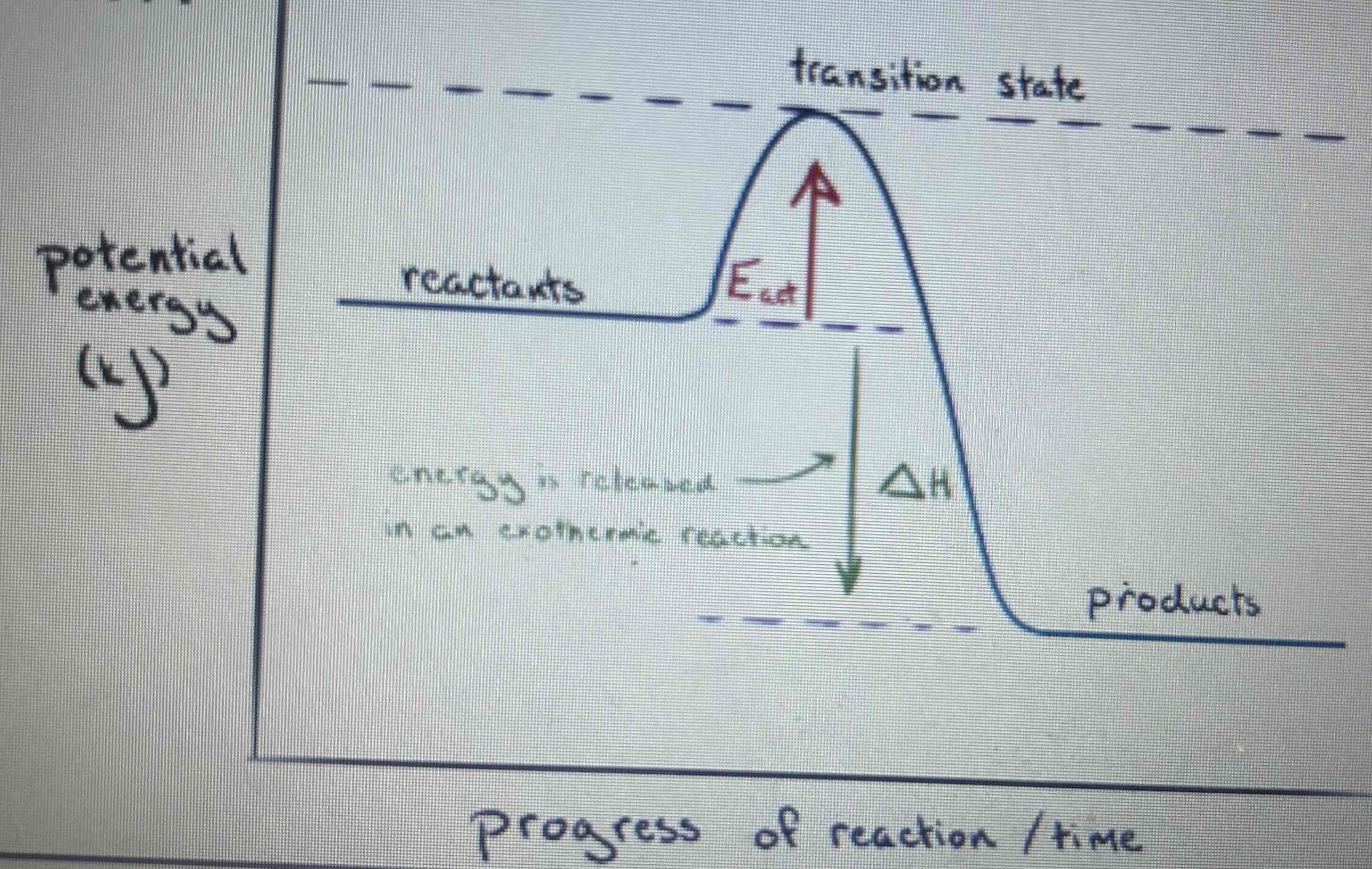
What are reaction profiles used for?
Used to show the relative energies of reactants and products, the activation energy and the overall energy change of a reaction
What is the activation energy?
The minimum amount of energy that particles must have to react
What is the reaction profile for an endothermic reaction
What is the reaction profile for an exothermic reaction?
How do you calculate bond energies?
•add together all the bond energies to break all the bonds in the reactants- this is the ‘energy in’
•add together the bond energies to form all the bonds in the products- this is the ‘energy out’
•calculate the energy change: energy in- energy out
If the overall enegrgy change js negative what does that indicate?
Exothermic - the energy is released to the surroundings, the bonds formed is larger than the bonds broken so exothermic
If the overall energy change is positive what does that indicate?
Endothermic- energy is taken in from surroundings. Bonds broken is larger than bonds formed so endothermic
What does the downwards arrow on a energy profile show
Energy given out
What does the upwards arrow on a energy profile show
Energy taken in
If the products have more energy than reactants in reaction profiles what does that mean?
Energy has been taken in as heat so reaction is endothermic
What does it mean if the products have less energy than reactants in a reaction profile
This “lost” energy has been given out as heat so reaction is exothermic