TRANSITION METALS
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
define a transition element.
a transition element is a d block element that forms at least one stable ion with a partially filled d subshell.
why are scandium and zinc not considered transition elements?
→ they form Sc3+ and Zn2+ which have no d electrons, and a completely filled d subshell respectively.
→ hence these ions do not have partially-filled d subshells
what are the exceptions of electronic configuration in transition metals?
→ Cr: [Ar] 3d^5 4s^1
→ Cu: [Ar] 3d^10 4s^1
more stable than 3d^4 4s² and 3d^9 4s²
→ In Cr, 3d and 4s orbitals are very close in energy. To minimise repulsion between paired 4s electrons, [Ar] 3d5 4s1 config is adopted rather than [Ar] 3d4 4s2 config
→ In Cu, full 3d subshell brings about more stability than a partial 3d subshell, due to symmetrical distribution of charge around the atom/ion
what are the physical & chemical properties of transition elements?
physical
hard, high density
good conductors of heat and electricity
high mp and bp
chemical
display variable oxidation states
form coloured compounds and ions
exhibit catalytic activity
form stable complexes
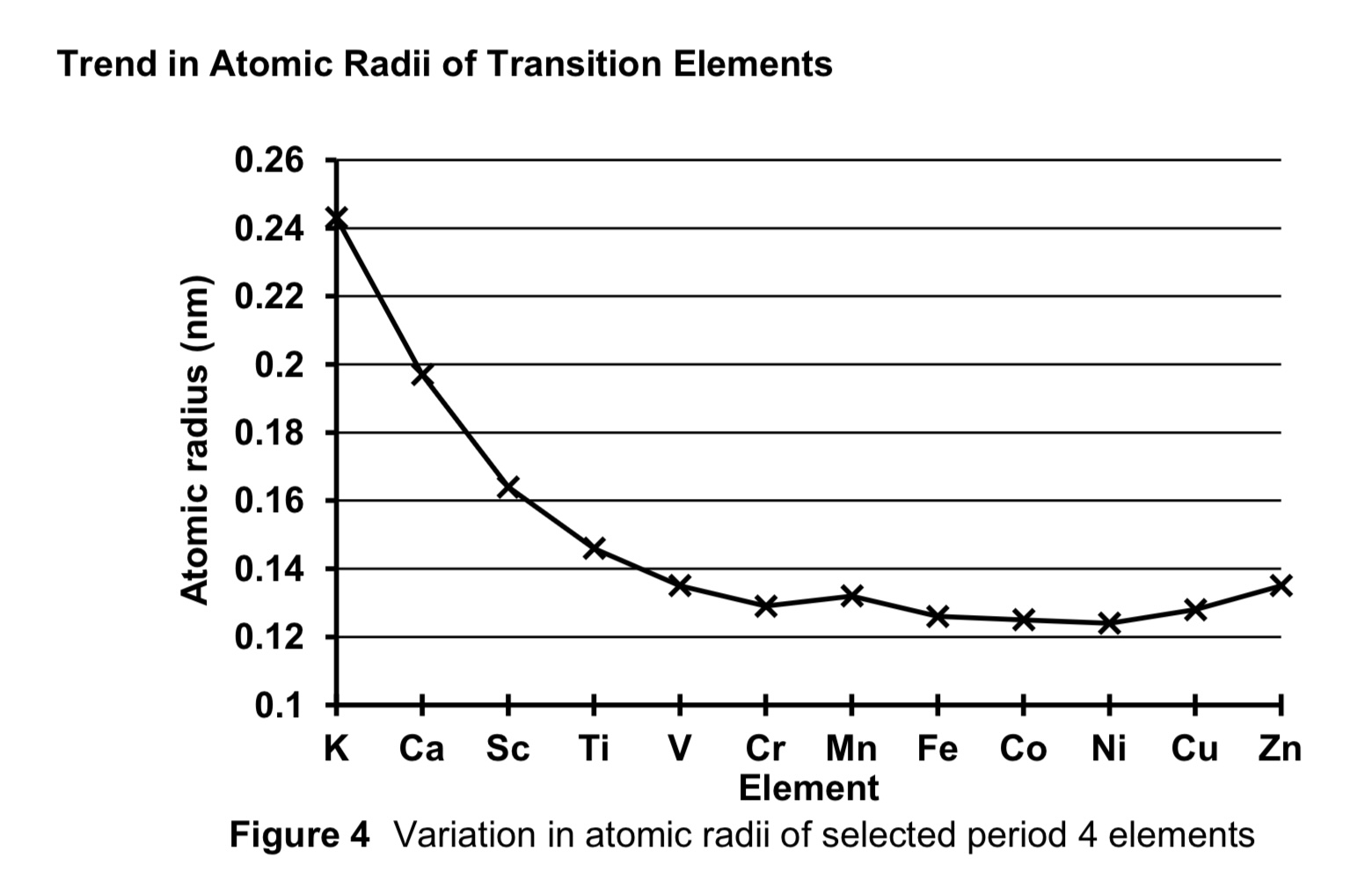
compare the atomic radii of first row transition elements against period 4 s block elements K and Ca.
from s block to d block, NC increases.
despite electrons being added to inner 3d orbitals, the 3d electrons are ineffective in shielding the 4s electrons from nucleus due to diffused 4 lobe shape of d orbitals
ENC increases, hence electrostatic foa between nucleus and valence electrons increase.
thus atomic radii of first row transition elements are smaller than those of period 4 s block elements.
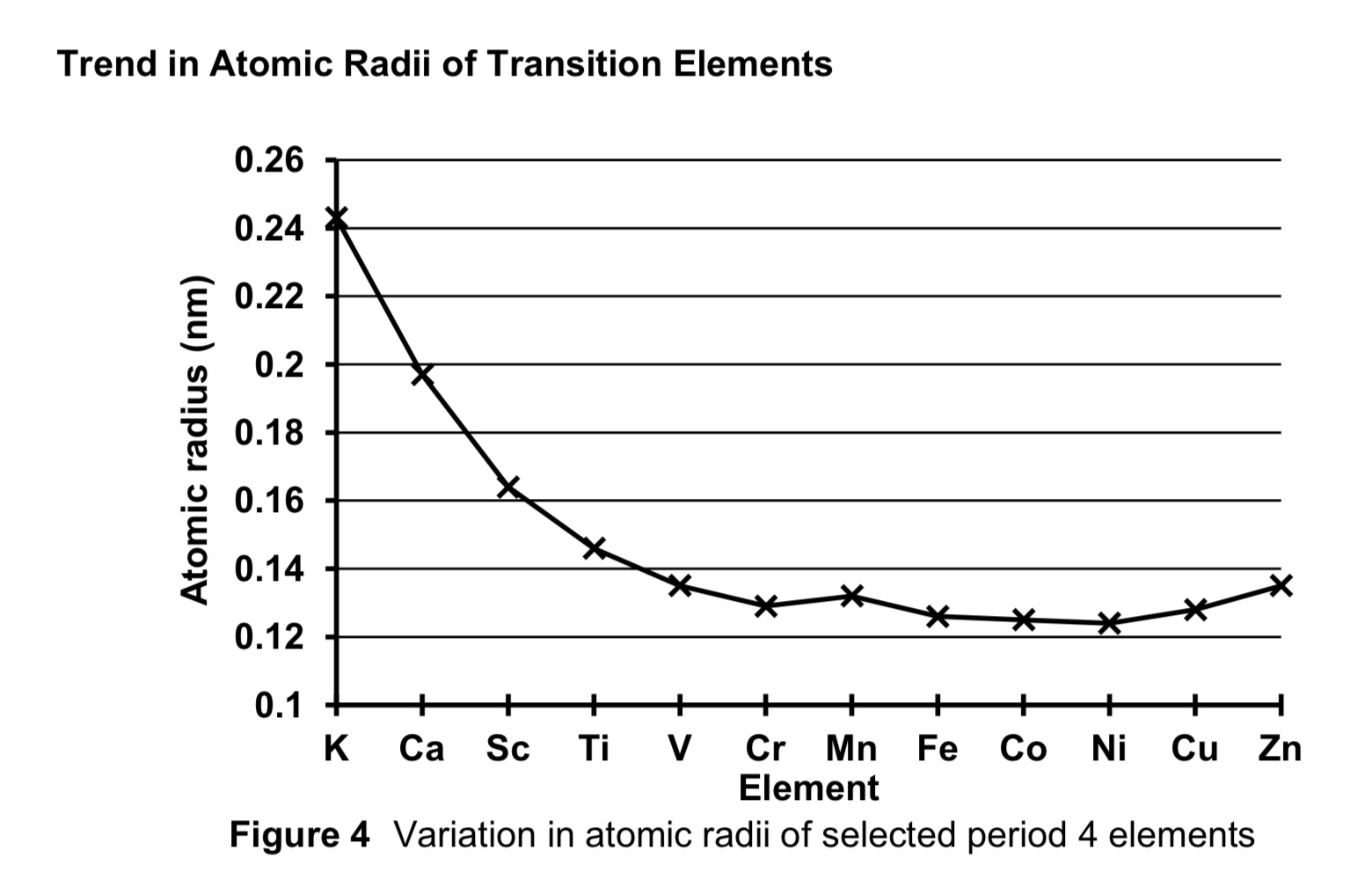
describe the trend of atomic radii of transition elements across the period.
across period 4 transition elements, NC increases.
each addition electron is added to the penultimate 3d subshell, SE also increases.
the increase in SE almost cancels out the increase in NC.
hence ENC increases only slightly
thus atomic radii is relatively constant/invariant across period 4 transition elements
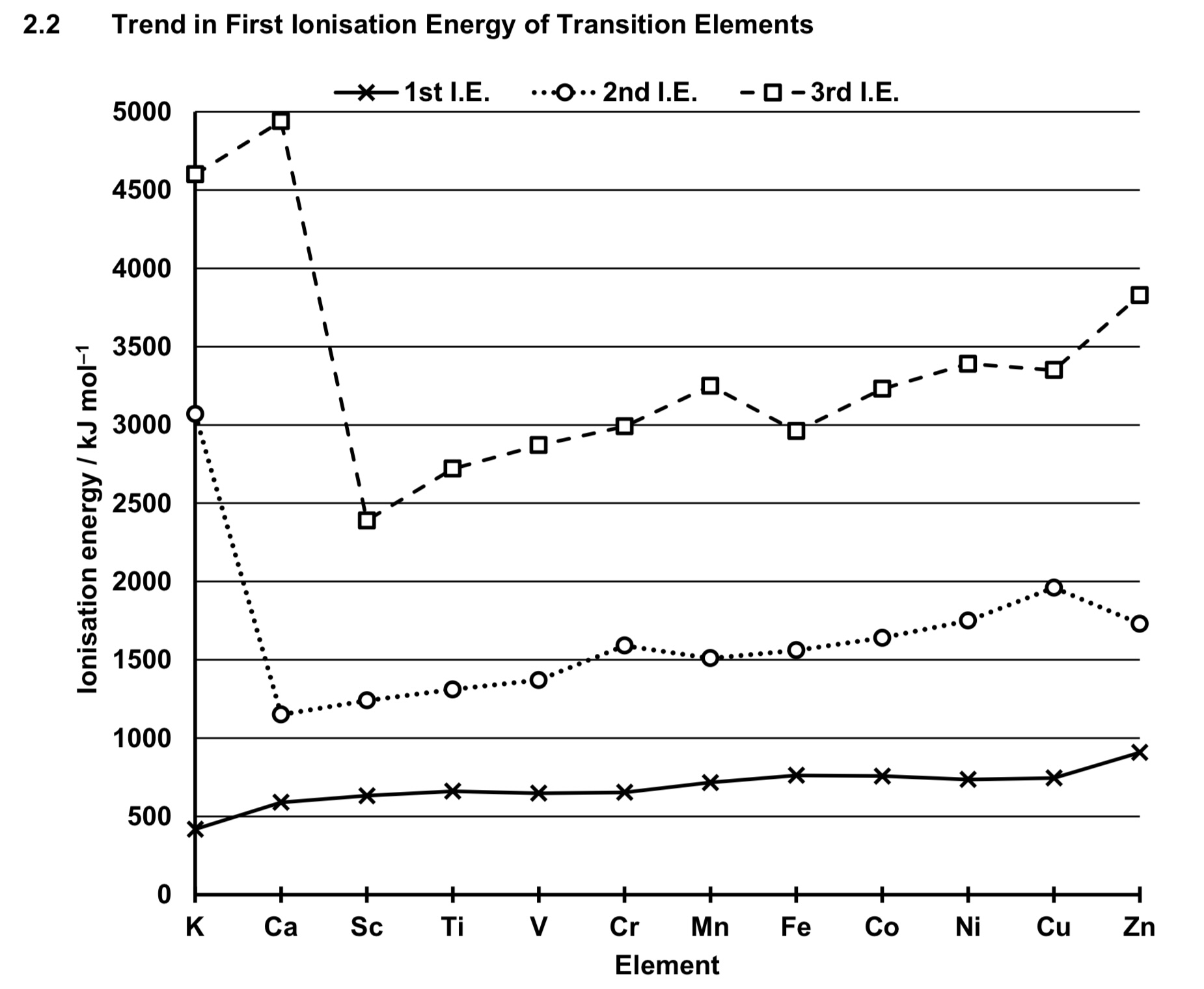
describe the trend of ionisation energy across period 4 transition elements.
across period 4 transition elements, NC increases.
since electrons are being added to inner 3d orbitals, SE also increases,
the increase in SE almost cancels out increase in NC,
thus ENC increases very gradually.
electrostatic foa between nucleus and valence electrons increase only slightly.
thus the energy required to remove the valence electrons increase only slightly.
HENCE FIRST IE OF TRANSITION ELEMENTS ARE RELATIVELY CONSTANT/INVARIANT ACROSS THE PERIOD
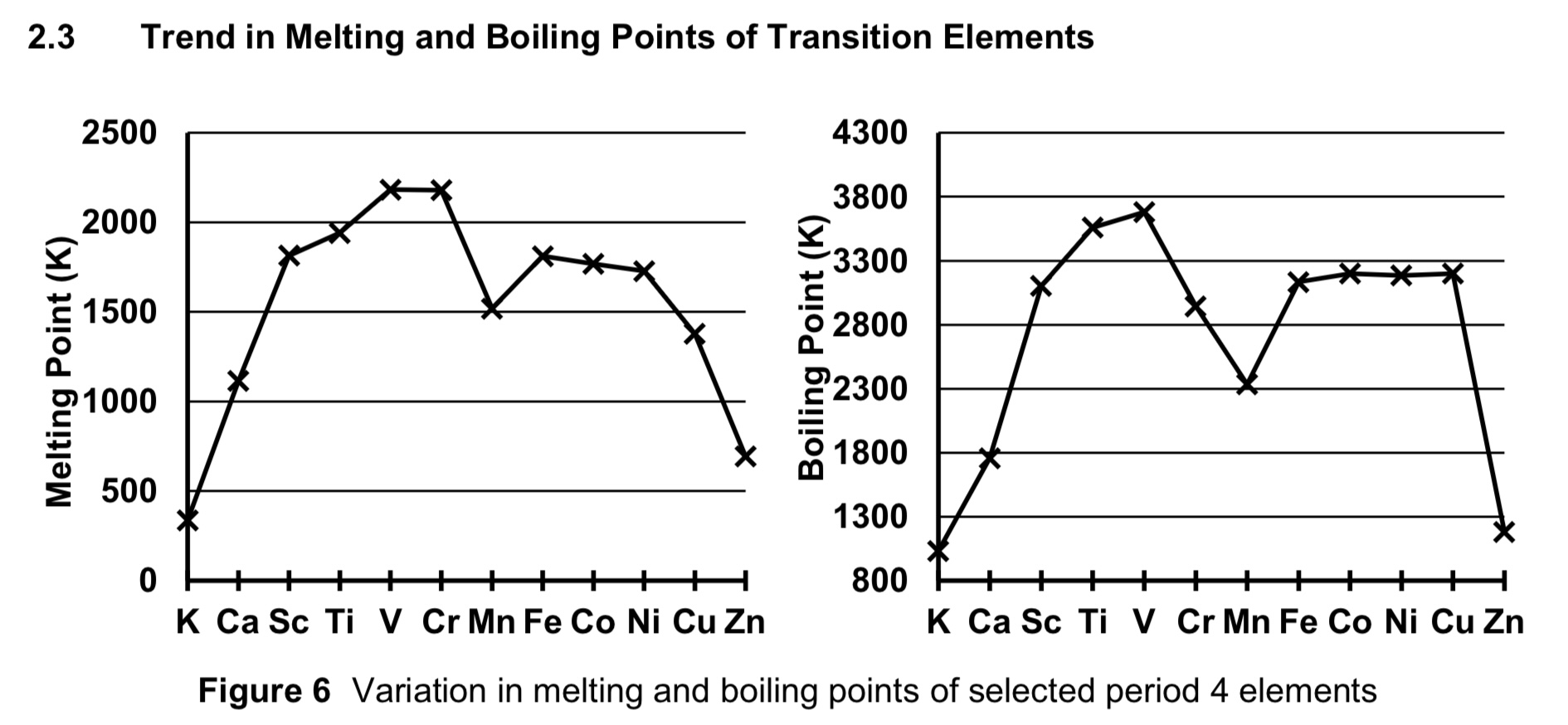
describe how transition metal melting and boiling points compare to that of s block elements.
both s block and transition elements have giant metallic lattice structures held together by strong metallic bonds
in transition elements, the sea of delocalised electrons is contributed by 3d and 4s electrons, since the energy level difference between the 3d and 4s orbitals is small.
in s block elements, only 1 or 2 4s electrons can be delocalised
HENCE, TRANSITION METAS HAVE HIGHER MP AND BP THAN THAT OF S BOCK ELEMENTS AS MORE ENERGY IS REQUIRED TO OVERCOME THE STRONGER METALLIC BONDS IN TRANSITION METALS
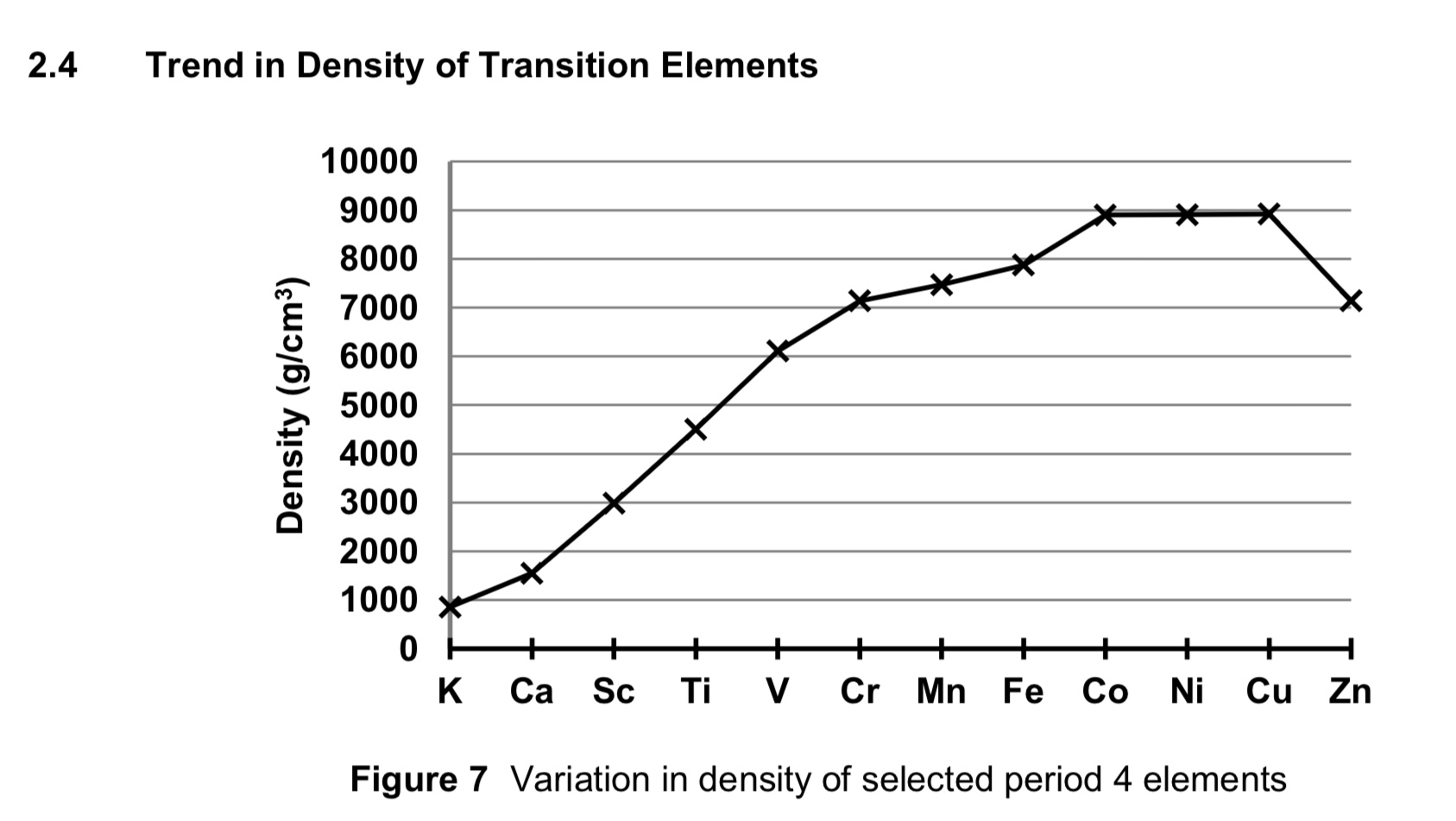
describe how density of transition metals compare to that of s block elements.
transition metal cations have relatively small atomic radii and higher atomic mass compared to s block elements.
transition elements have more closely packed structures due to their stronger metallic bonding compared to s block elements.
more atoms of d block elements are packed in a unit volume compared to the s block elements within the same period.
hence transition elements are denser than s block elements.
list the chemical properties of transition elements that s block elements do not have.
1) displaying variable oxidation states
2) forming stable complexes
3) forming coloured compounds and ions
4) exhibiting catalytic properties
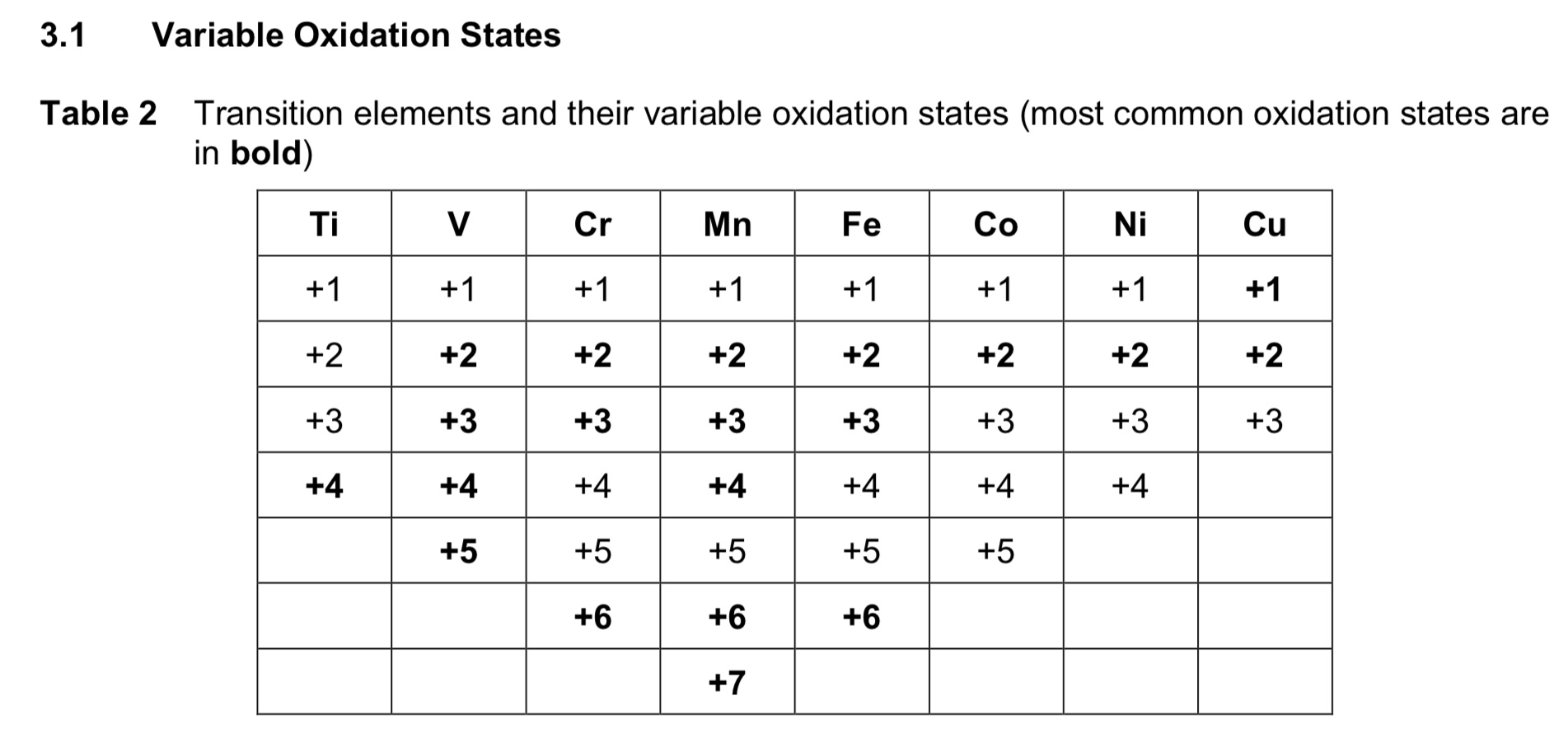
how do you calculate the maximum oxidation state of a transition element?
no. of 4s electrons + no. of unpaired 3d electrons
why can transition metals display variable oxidation states?
3d and 4s orbitals are close in energies,
hence diff no. of 3d and 4s electrons can be lost to form stable ions or
utilised in bonding to form compounds of diff ox states
predicting common + max oxidation states of transition metals.
Mn is the turning point. increase from Ti 4+ to Mn 7+, then decrease to Cu 3+.
How do compounds of transition elements differ in terms of character?
→ Compounds of transition elements in low oxidation states are usually ionic, thus basic. eg MnO, CrO (Mn2+, Cr2+)
→ Compounds of transition elements in high oxidation states are usually covalent, thus acidic. eg Mn2O7, MnO4²- (Mn7+, Mn6+)
→ Oxides of transition elements in intermediate oxidation states are usually amphoteric eg Cr2O3 (Cr3+)
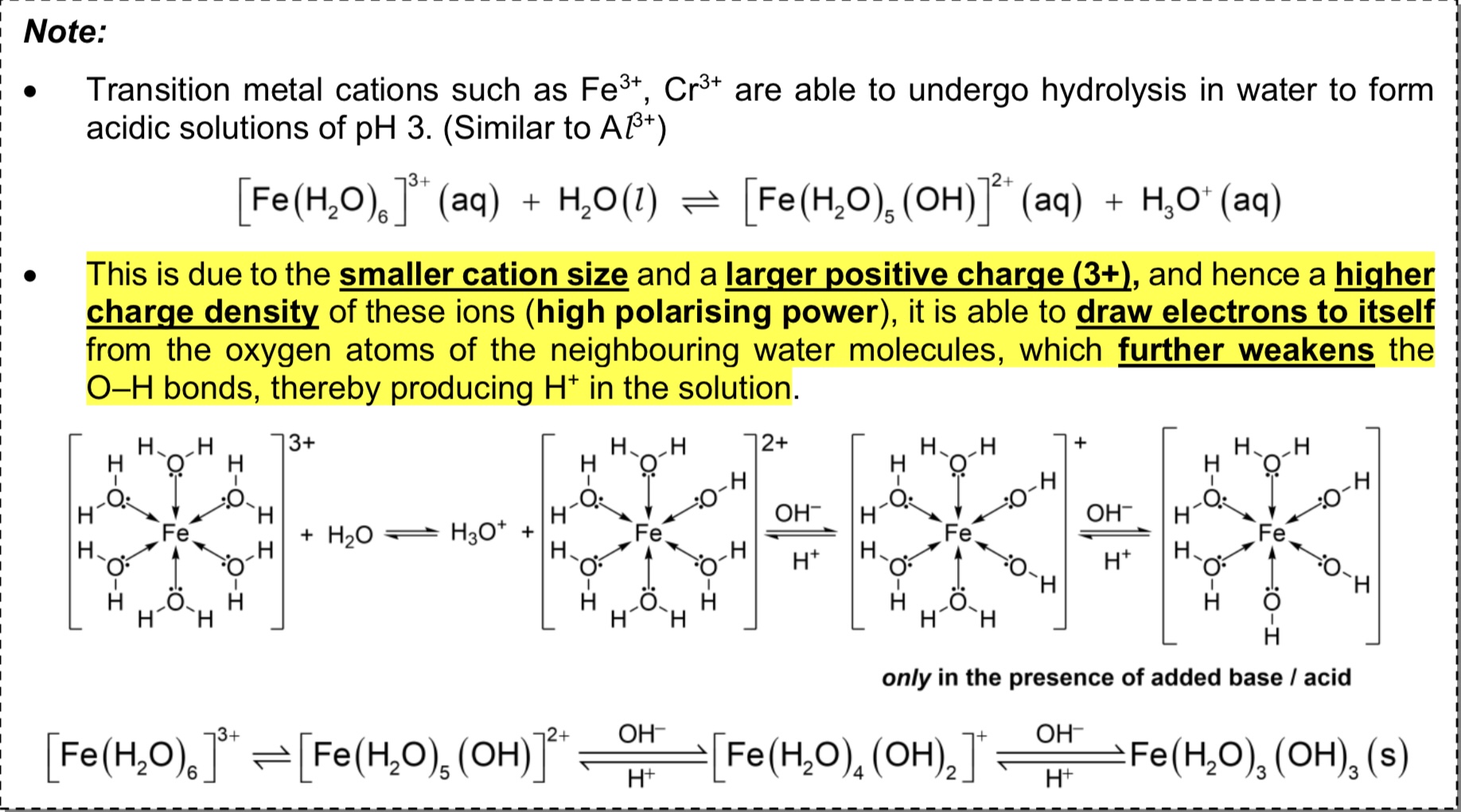
Why are solutions of Fe3+, Cr3+, or Al3+ acidic?
→ The small cationic size and large positive charge leads these metal cations to have higher charge density.
→ Hence these cations can draw electrons to itself from O atoms of H2O molecules, weakening the O-H bonds and producing H+ in the solution.
→ These cations can undergo hydrolysis in water to form acidic solutions of pH3.
describe and explain the trend of the stability of +2 oxidation states across the d-block.
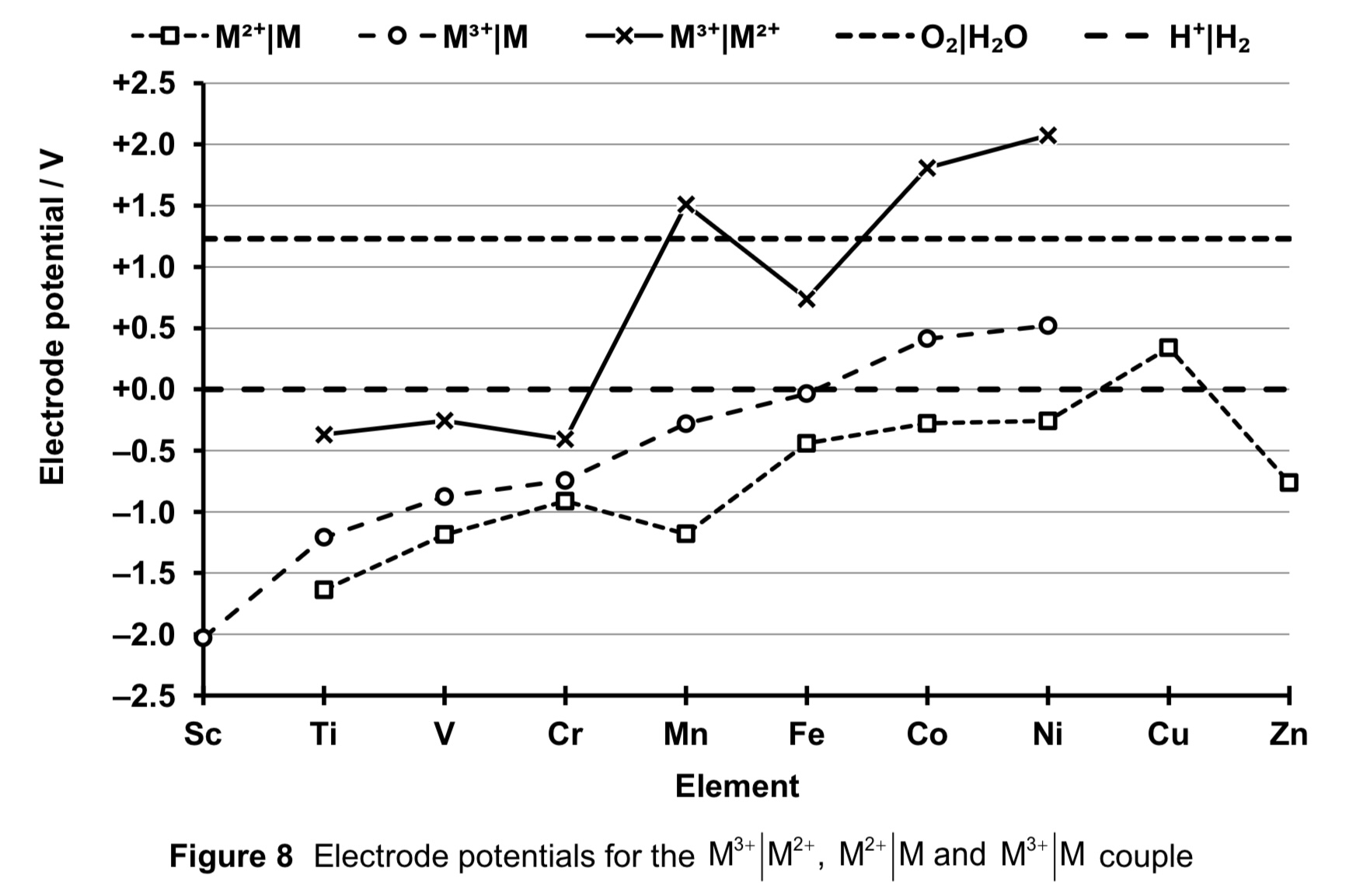
With the exception of Cu2+, M2+(aq) is more stable relative to M(s) as seen from the negative E (M2+ (aq) | M (aq) ) for the other elements, which means M2+ is less likely to undergo reduction.
Since E (M2+ (aq) | M (aq) ) is negative compared to E (H+ (aq) | H2 (g) ) = 0.00 V, all the metals except Cu are expected to react with aqueous acids to give M2+(aq), although the M2+(aq) ion can undergo further oxidation to higher oxidation states.
Generally, the value of E (M2+ (aq) | M (aq) ) becomes increasingly less negative from left to right of the d-block. This would mean that the oxidation of the metal to form M2+ becomes decreasingly less spontaneous from left to right of the d-block.
In short, M2+(aq) is increasingly less stable relative to M(s) from left to right of the d-block, oxidation of M to M2+ is decreasingly likely to occur, i.e. the metals are less reactive towards oxidation.
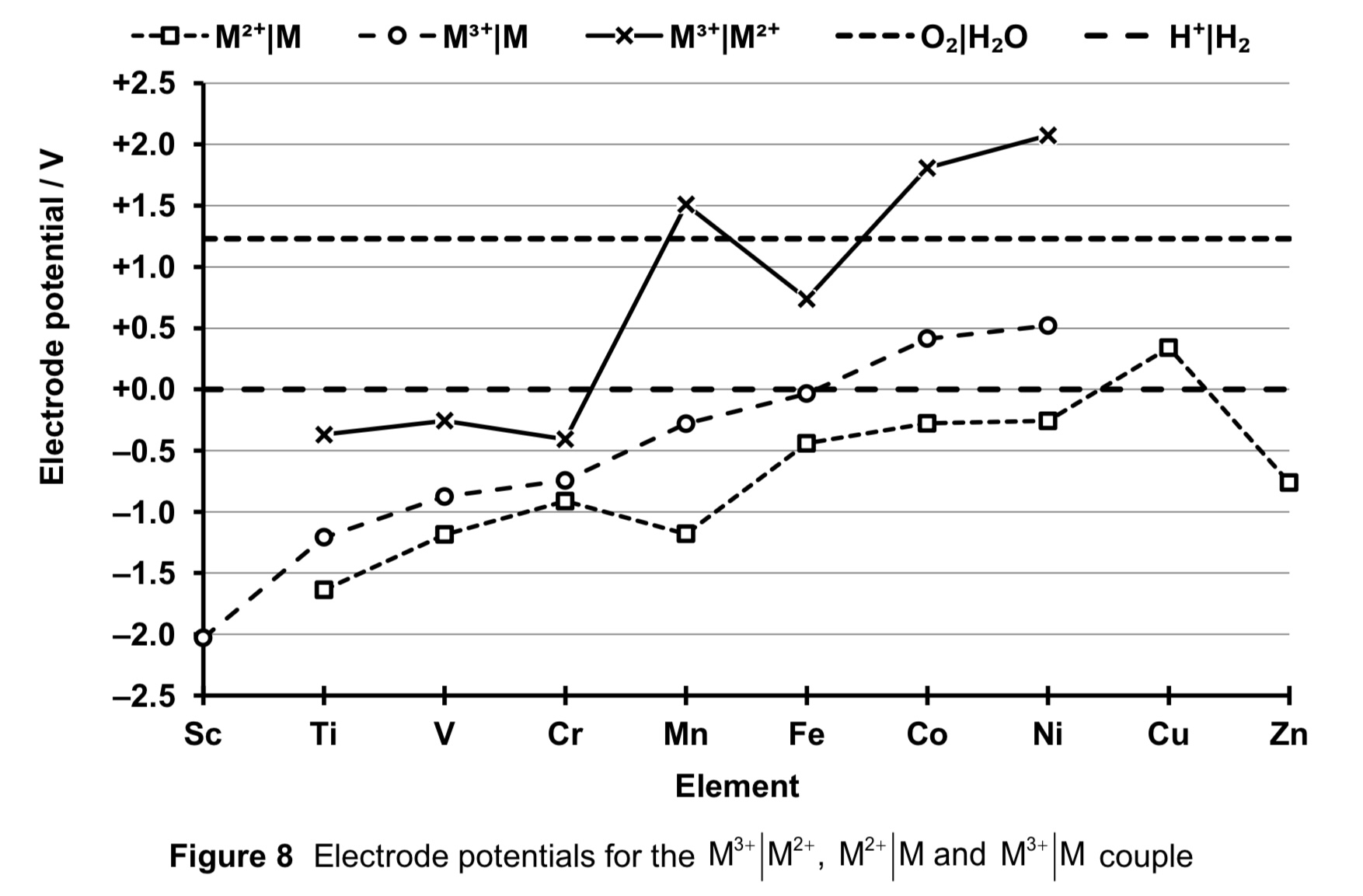
describe and explain the trend of stabilities for +3 oxidation state across the d-block.
With the exception of Co3+ and Ni3+, M3+ (aq) is more stable relative to M(s) as seen from the negative E (M3+ (aq) | M (aq) ) for the elements.
Generally, the value of E (M3+ (aq) | M (aq) ) becomes increasingly less negative, meaning M3+ (aq) is increasingly less stable relative to M(s). Oxidation of M to M3+ is decreasingly likely to occur and the metals are less reactive towards oxidation.
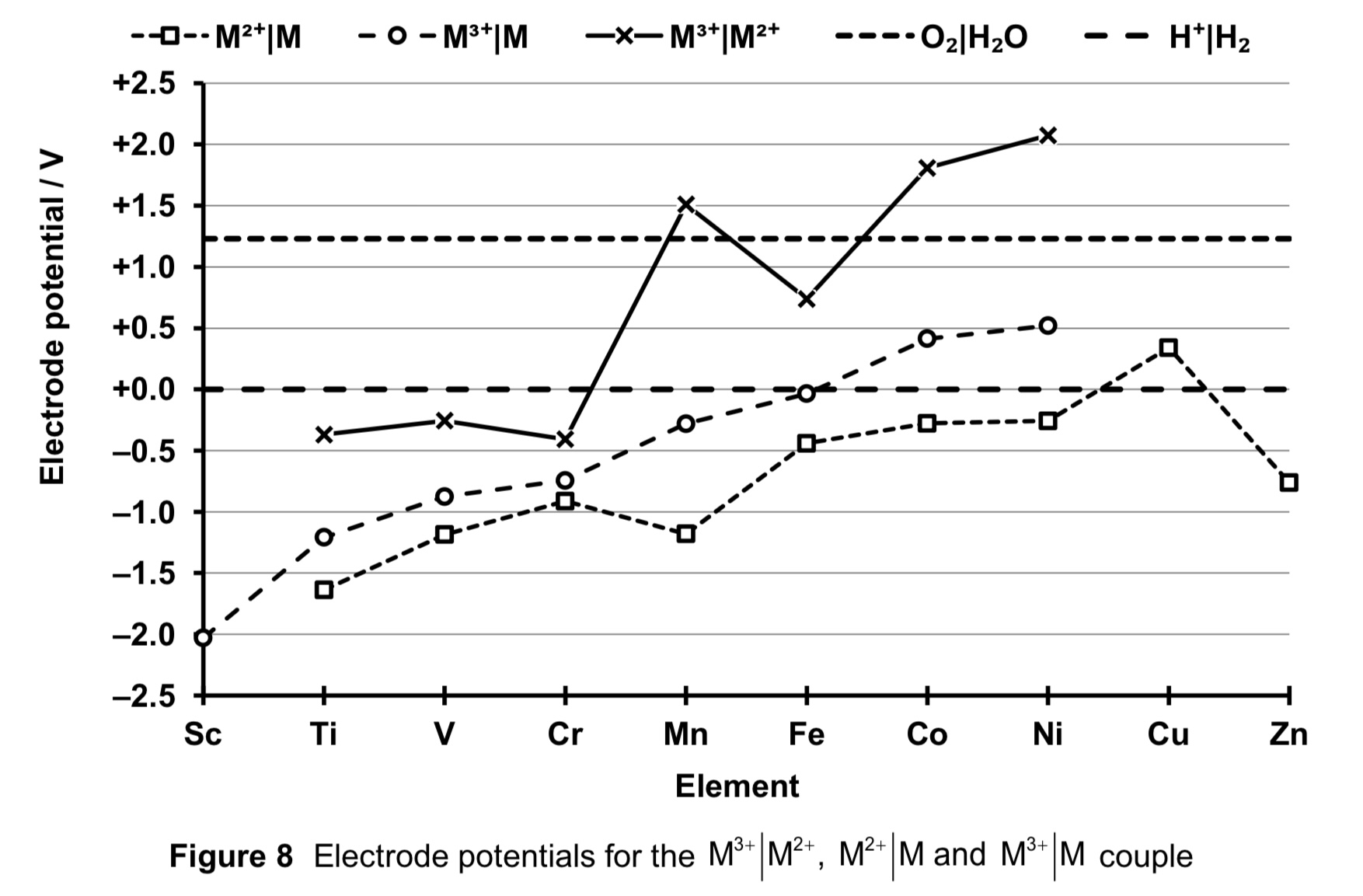
compare the stabilities of +2 and +3 oxidation state across the d-block.
generally, both M2+ and M3+ decrease in stability across the series.
however, stability of M3+ decreases faster than that of M2+, hence stability of +2 oxidation states relative to the 3+ oxidation state increases across the series. this is seen in the general increasingly less negative E (M3+ (aq) | M2+ (aq) ), indicating that reduction of M3+ to M2+ is increasingly likely to occur.
across the period, ENC increases for M2+ ions as NC increases while SE remains relatively constant.
the electrostatic foa between nucleus and valence d electron to be removed increases.
thus there is increasing difficulty of removing a d electron and the stability of the +2 oxidation state increases across the series
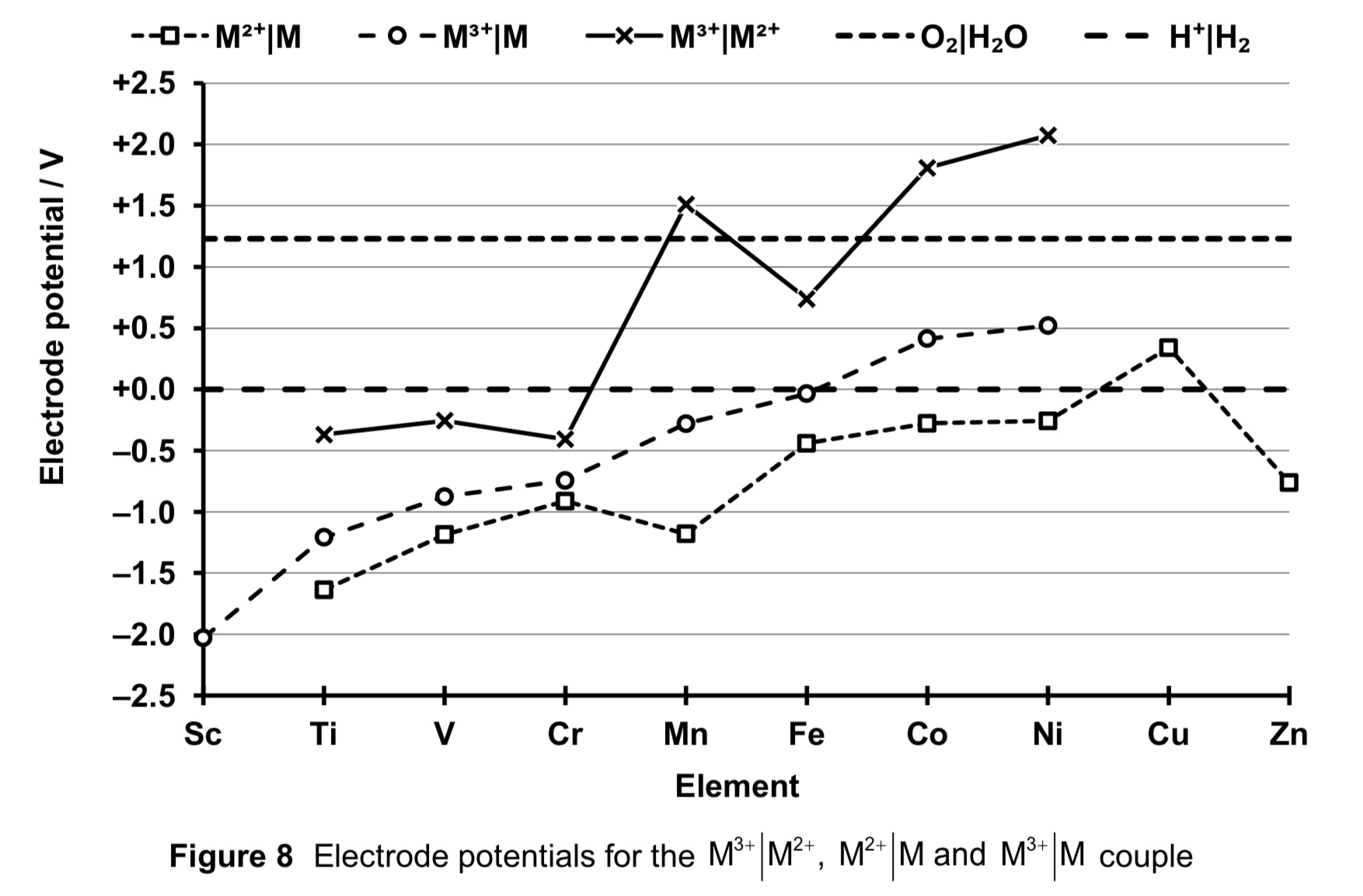
what do negative E values for Ti, V and Cr indicate? conversely, what do positive E values for Mn to Ni indicate?
→ they indicate that reduction M3+ (aq) + e → M2+ (aq) is NOT thermodynamically favourable
→ hence M3+ (aq) more stable wrt M2+
→ M2+(aq) would be easily oxidised, hence M2+ is a good RA
→ they indicate that reduction M3+ (aq) + e →M2+ (aq) IS thermodynamically favourable
→ hence M2+ (aq) more stable wrt M3+ (aq)
→ M3+ would be easily reduced, hence M3+ is a good OA
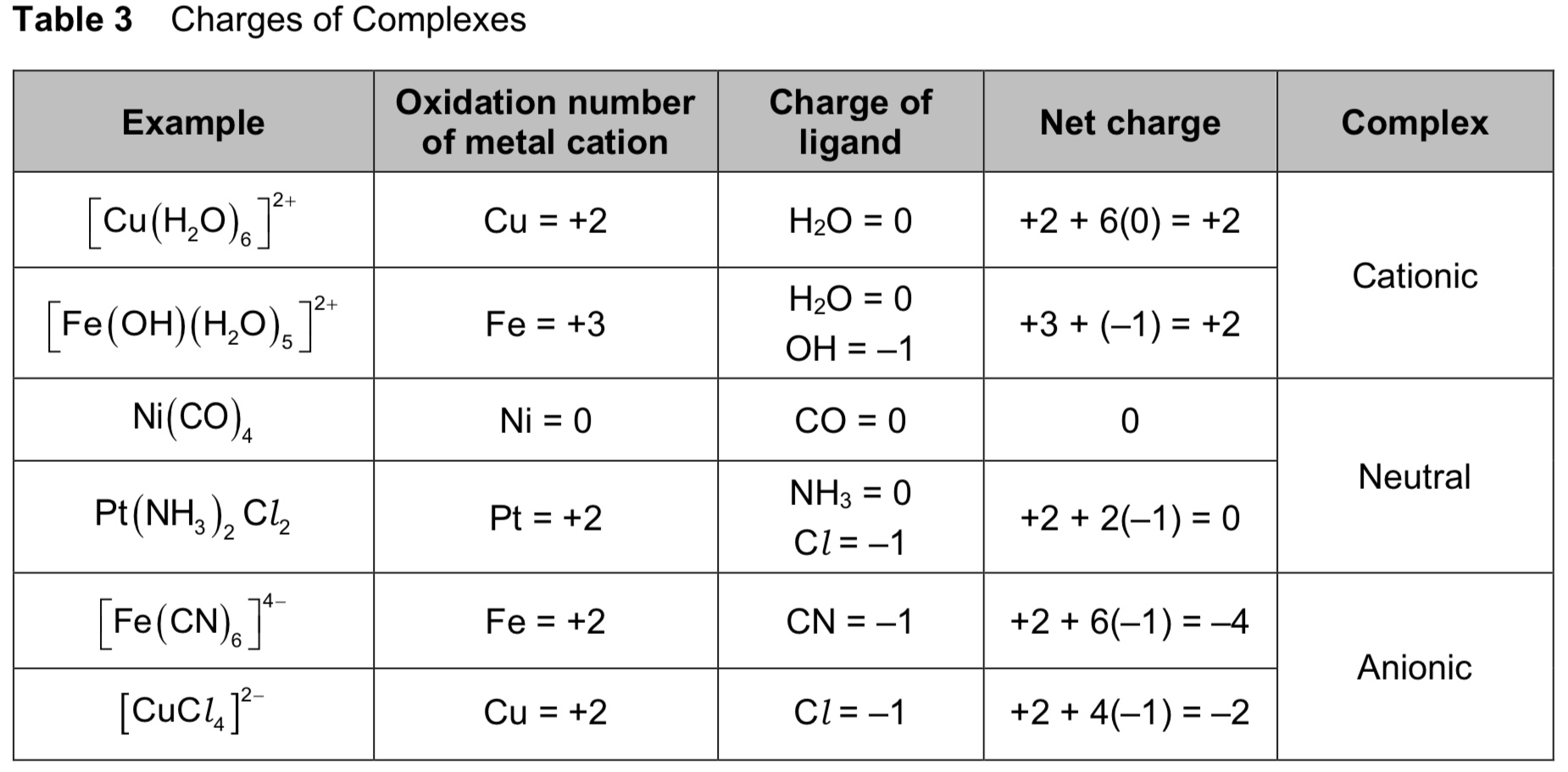
define “transition metal complex”. define “ligand”.
a complex consists of a central metal atom or ion surrounded by other ions or molecules, called ligands, bonded to the central atom/ion by dative covalent bonds.
a ligand is defined as a molecule/ion which contains at least 1 lone pair of electrons available for forming dative/coordinate bonds with the central metal atom/ion.
why do transition metal cations tend to form complexes?
due to their relatively small size and high charge, transition metal ions have high charge density and high polarising power to attract ligands
transition metal ions also have low-lying vacant d orbitals which can accept lone pairs of electrons from ligands via dative covalent bonds
what is coordination number, and denticity?
→ coordination number is defined as total number of dative bonds formed between ligands and central metal atom/ion
→ denticity refers to the no. of dative bonds 1 ligands can form with the central metal atom/ion. eg EDTA4- is hexadentate as it forms 6 dative bonds, NH3 is monodentate as it forms 1 dative bond each
NOTE: each atom can donate one lone pair of electrons. but in a molecule, more than one atom can form dative bonds
eg of monodentate ligands (must know): NH3, H2O, CN-, Cl-, F-, SCN-. for SCN-, lone pairs on either S or N can be used for formation of dative bonds, due to resonance within the ion.
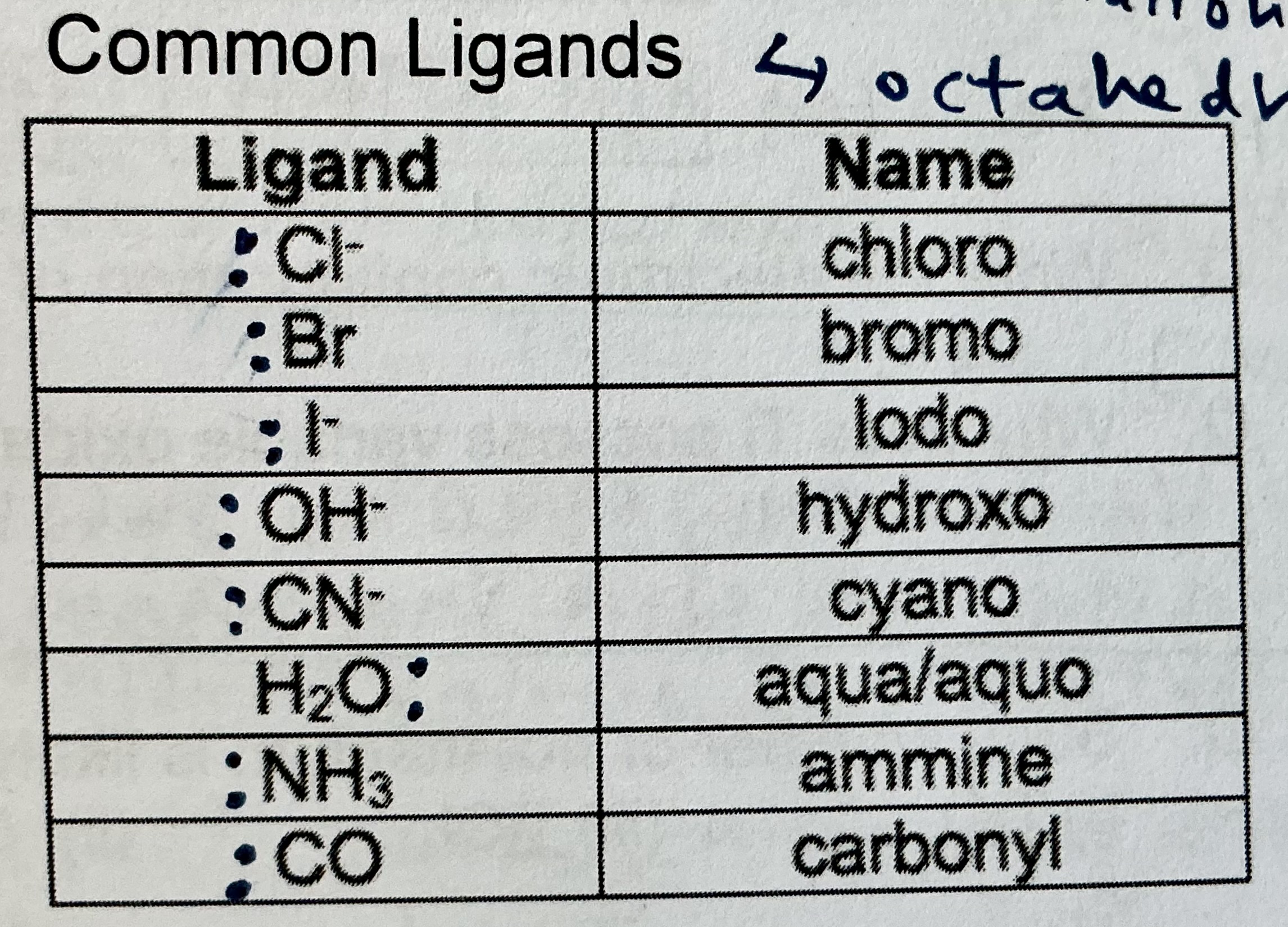
why may polydentate ligands be used for complex formation?
→ complexes formed with polydentate ligands are stable. eg EDTA stabilised metal cations, making them unreactive
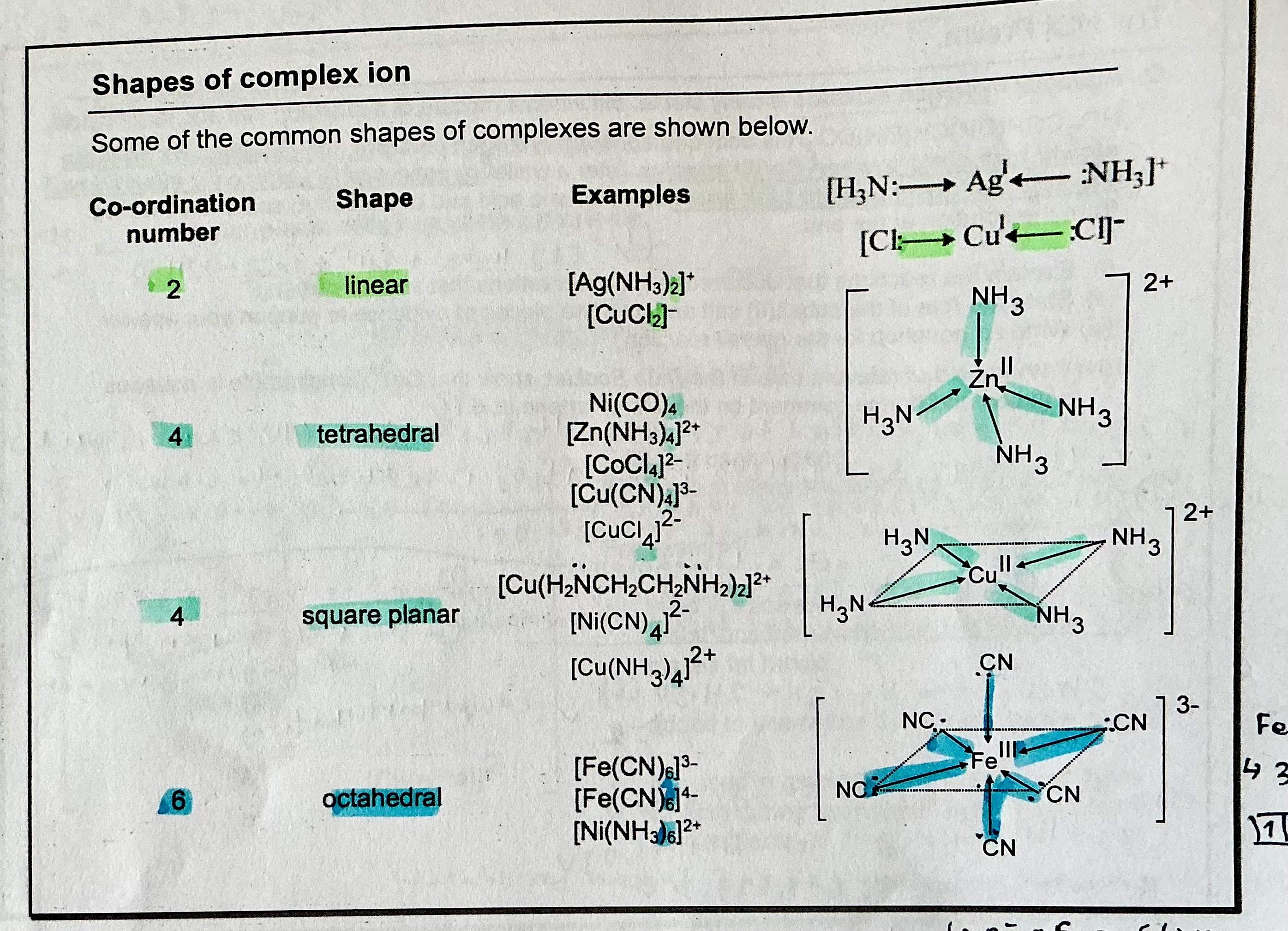
shapes of complexes
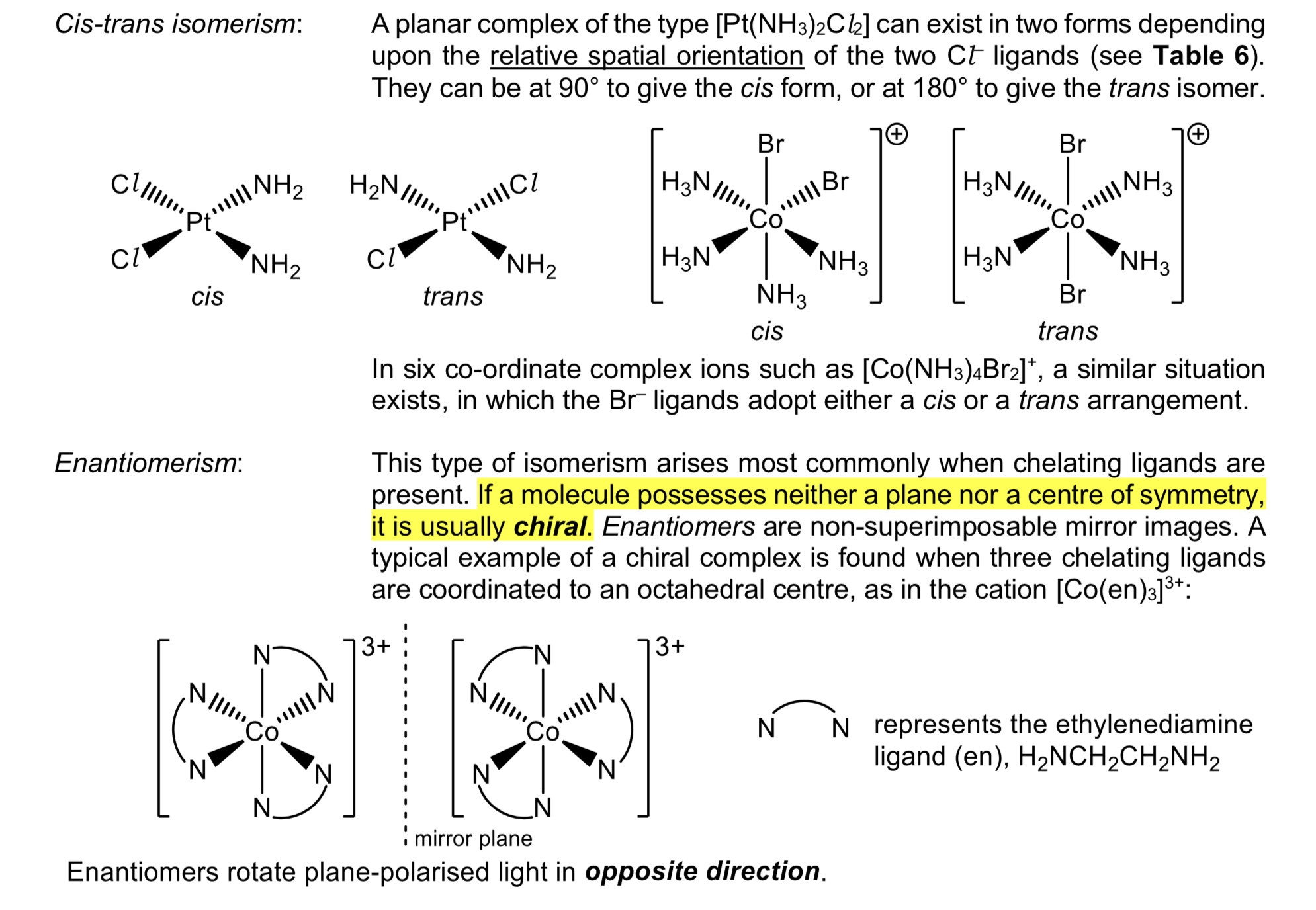
cis-trans isomerism and enantiomerism in complexes
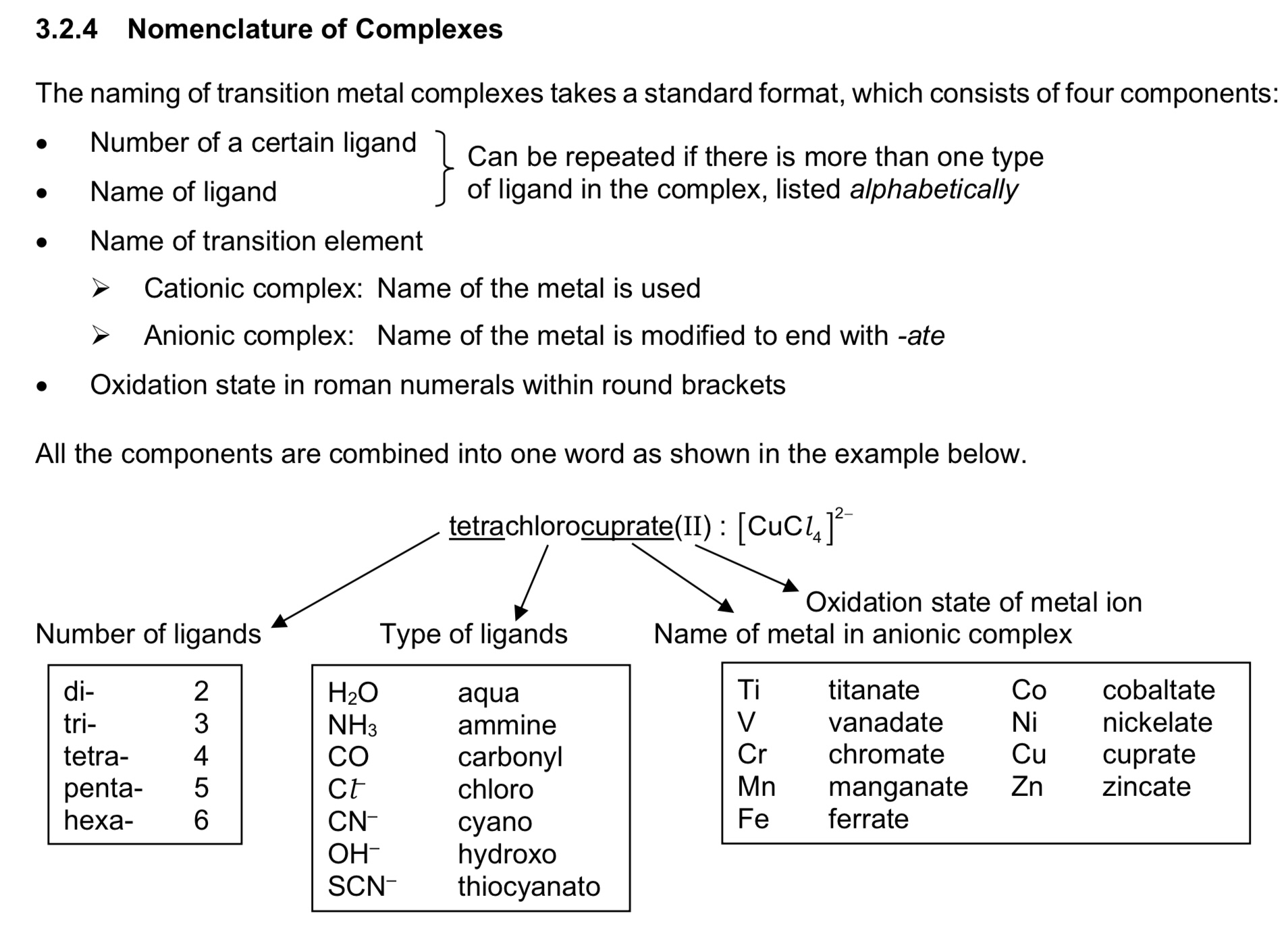
nomenclature of complexes
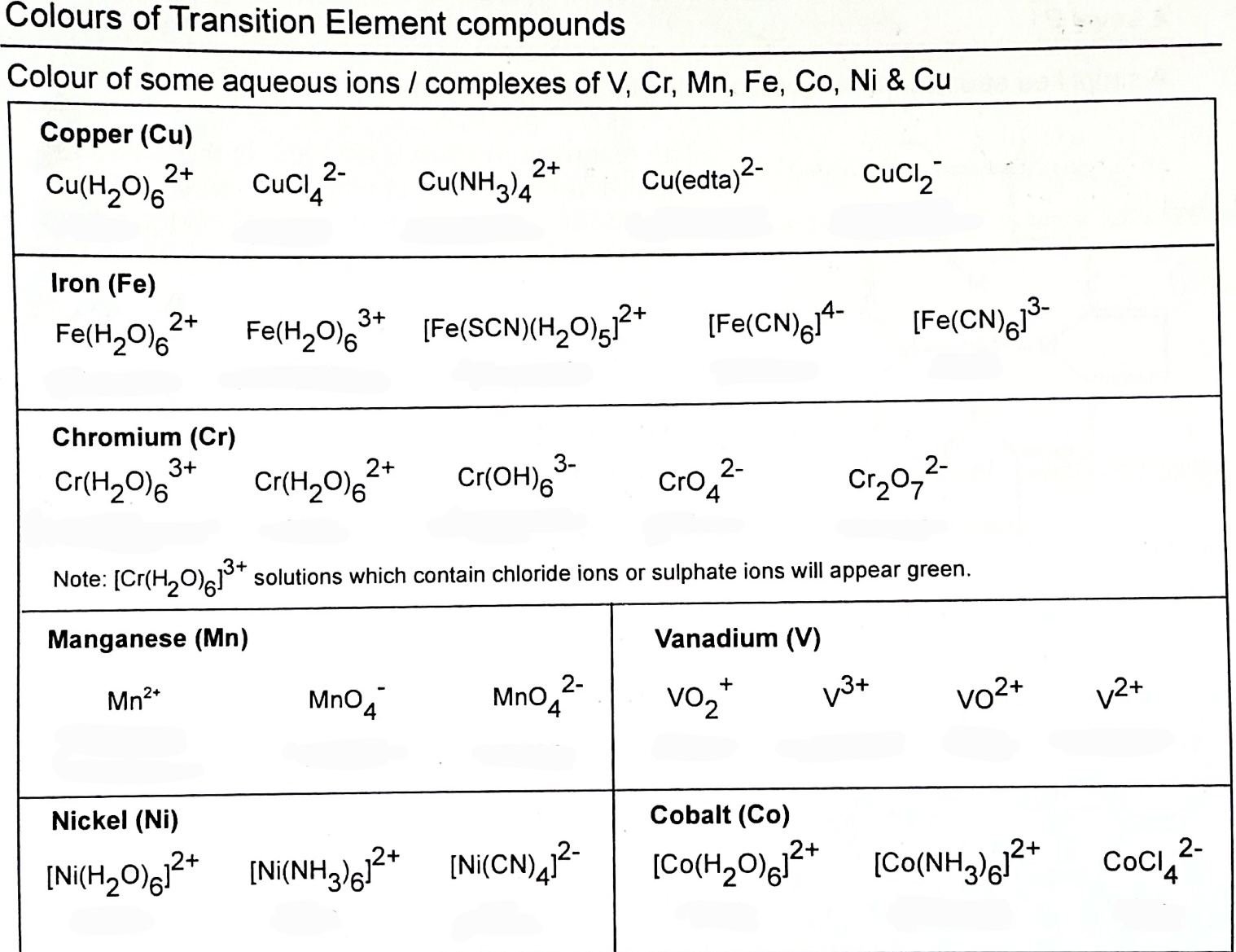
list the colours of the compounds
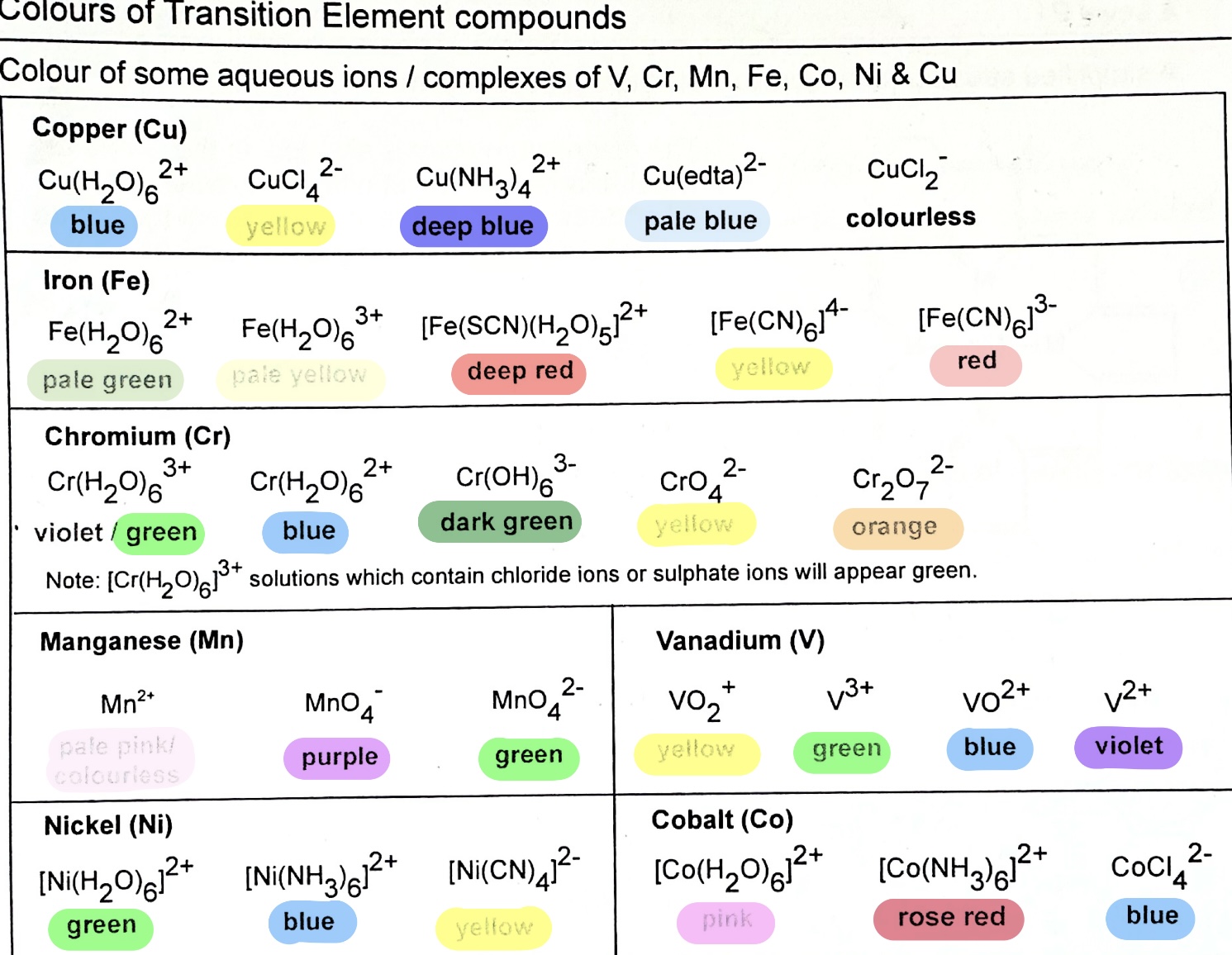
Why are compounds of transition elements usually coloured?
PRE-REQUISITE: FOR D-D TRANSITION TO TAKE PLACE, at least one of the d orbitals must be occupied by an electron, and d subshell must not be fully-filled
→ In the presence of ligands, the 3d orbitals of the transition elements are split into 2 different energy levels.
→ An electron in the lower energy level can absorb visible light of a certain wavelength and is promoted to a higher energy d orbitals. This is called d-d transition.
→ The colour observed is the complementary colour absorbed.
note: extent of d-d splitting depends on
→ type of ligands (stronger ligand leads to bigger energy gap)
→ oxidation state (no. of 3d electrons)
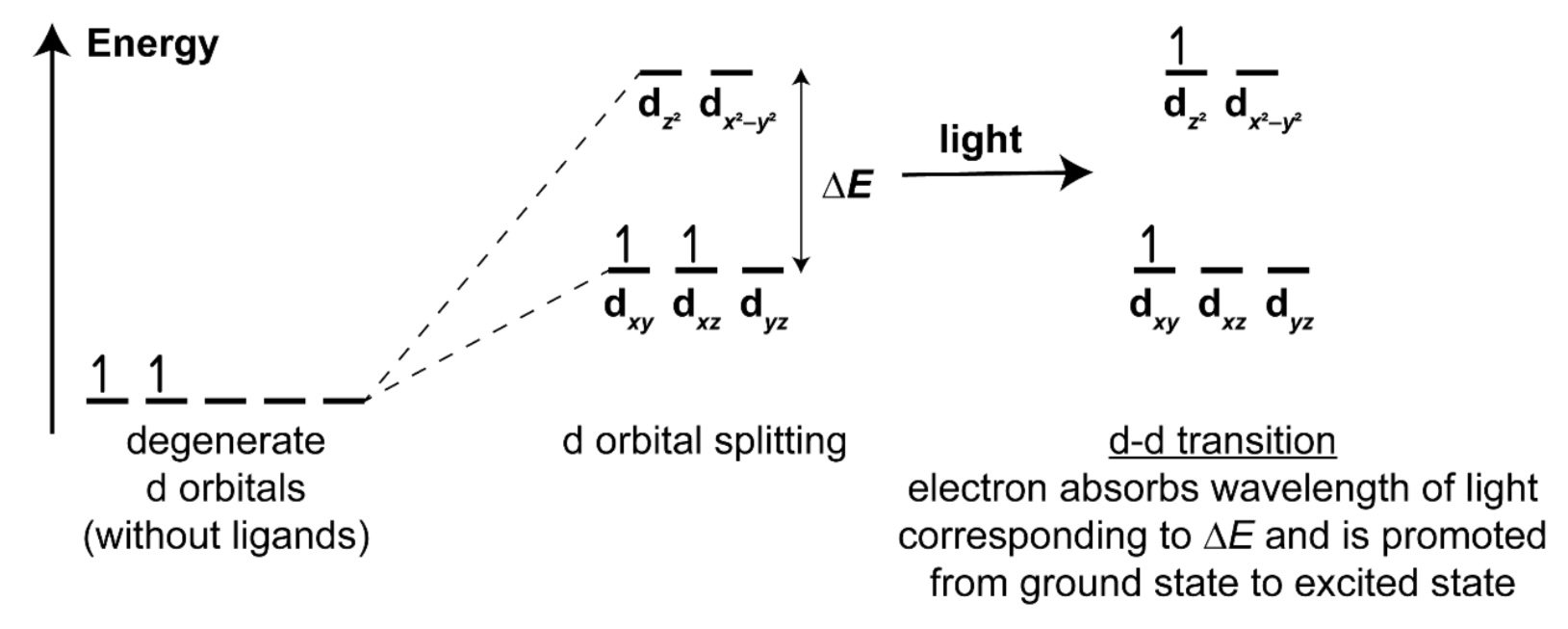
how to determine what colour is observed from complex of transition element?
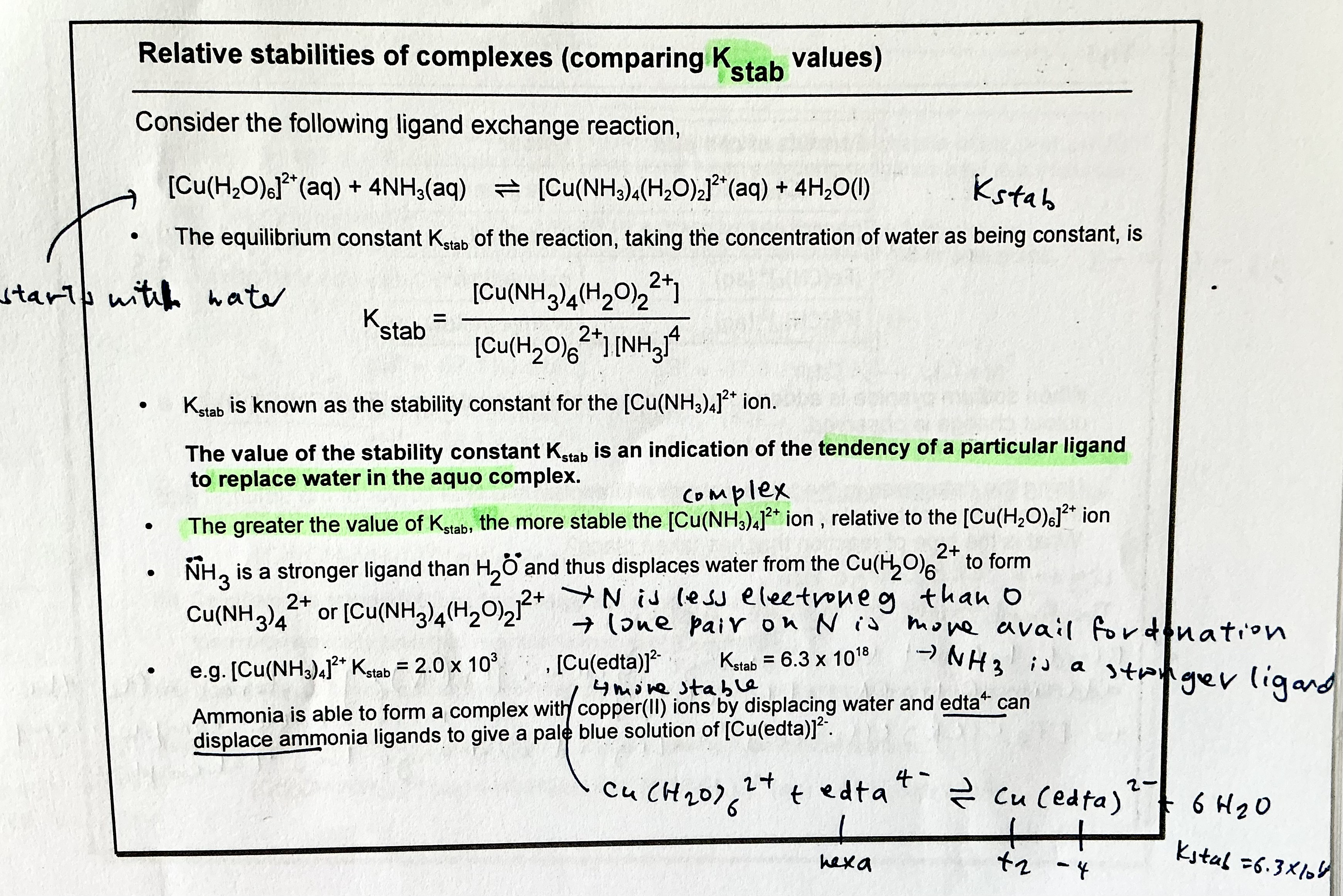
How to determine relative stability of complexes?
factors affecting energy gap between 3d orbitals in a complex
nature of ligands
weak field ligands absorb longer lambda, leading to smaller E.
strong field ligands absorb shorter lambda, leading to larger E.
identity of transition metal and its oxidation state (FYI)
generally the energy gap increases with oxidation state. the higher the charge density of metal ion, the stronger the attraction for the ligands, hence stronger the repulsion, leading to larger E.
shape of complex (FYI)
what factors affect ligand exchange?
strength of ligands
stronger ligands replace weaker ligands
conc of ligands
in the presence of a large conc of diff ligands, ligand exchange can occur for a complex ion
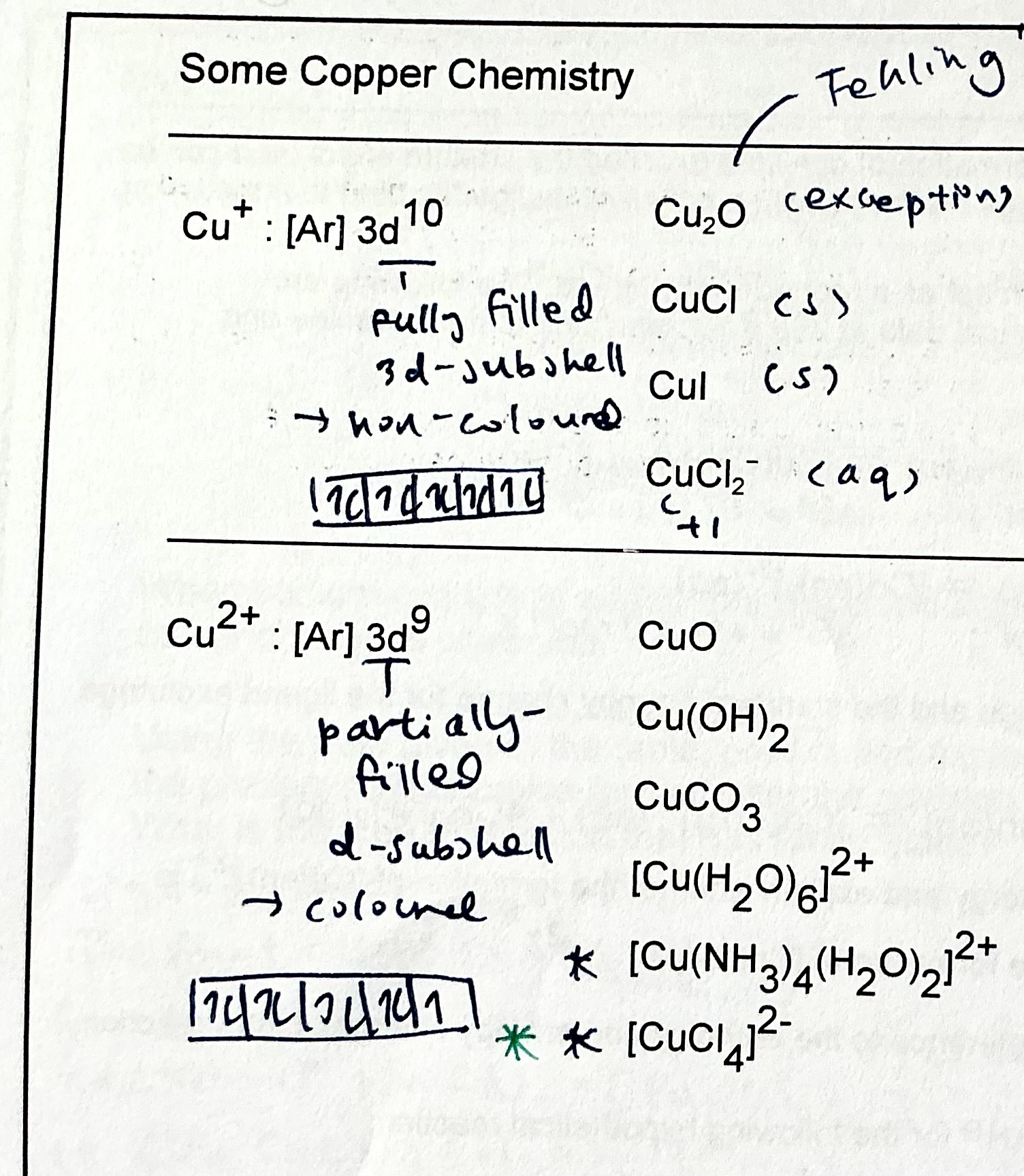
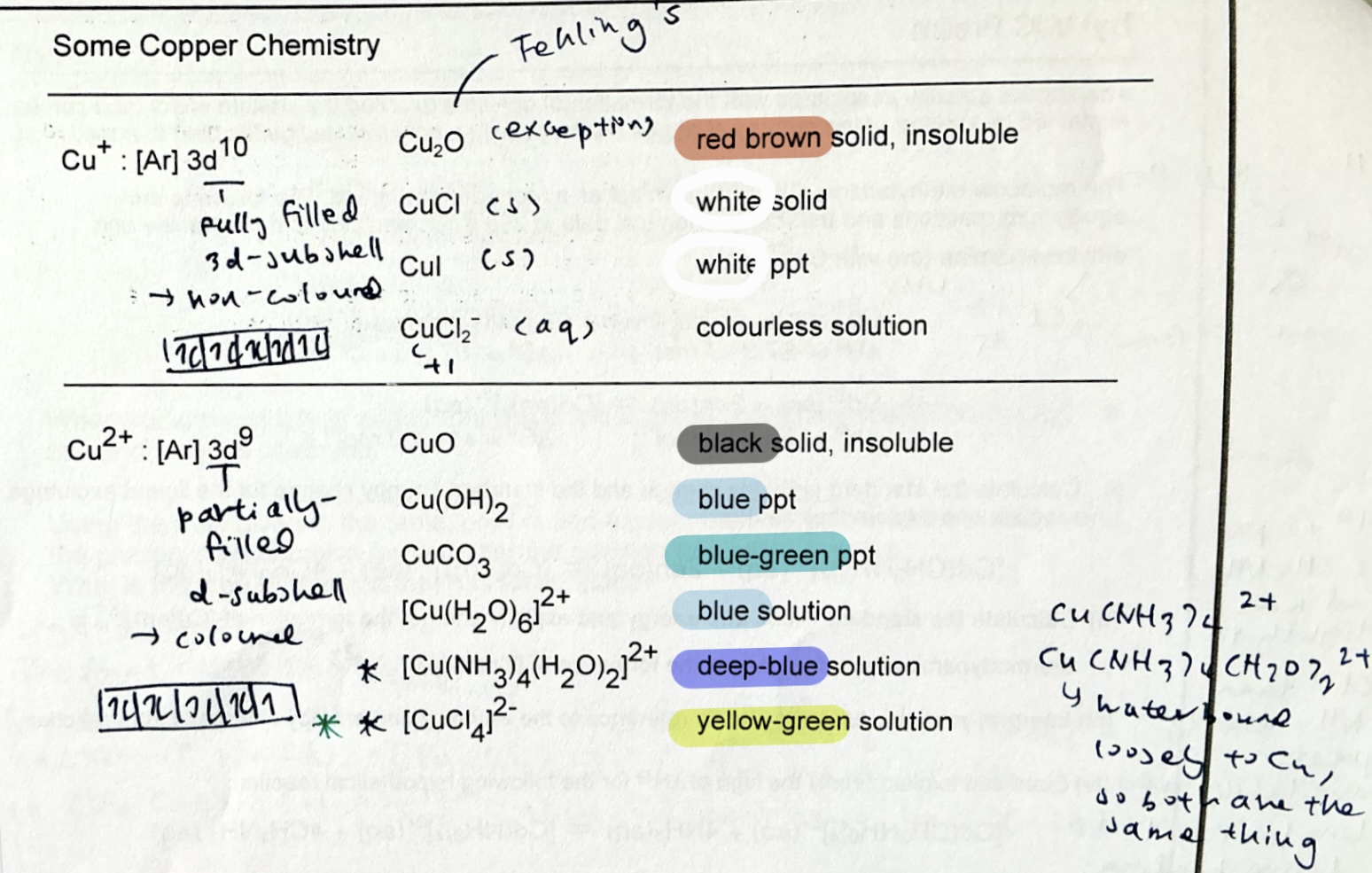
list colours of Cu compounds.
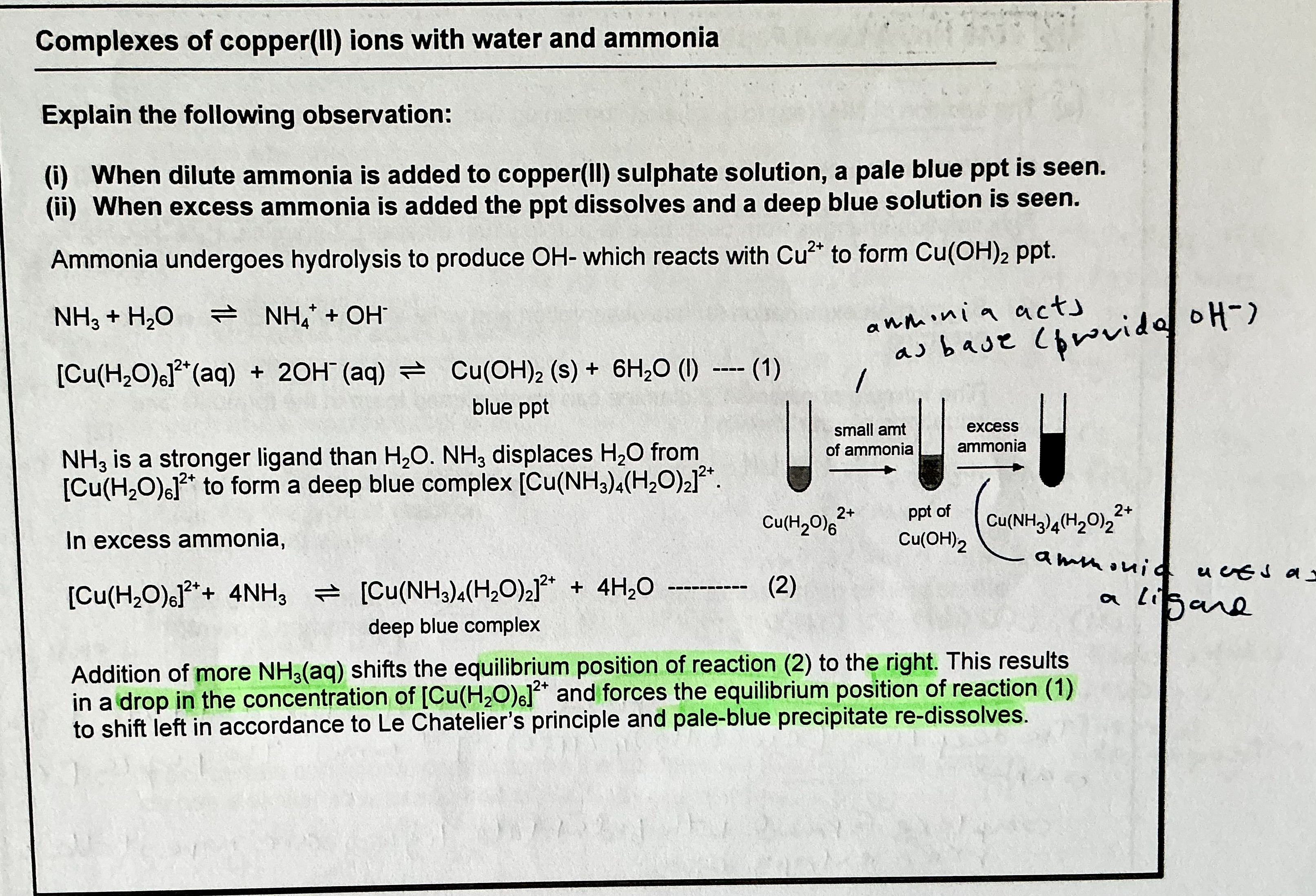
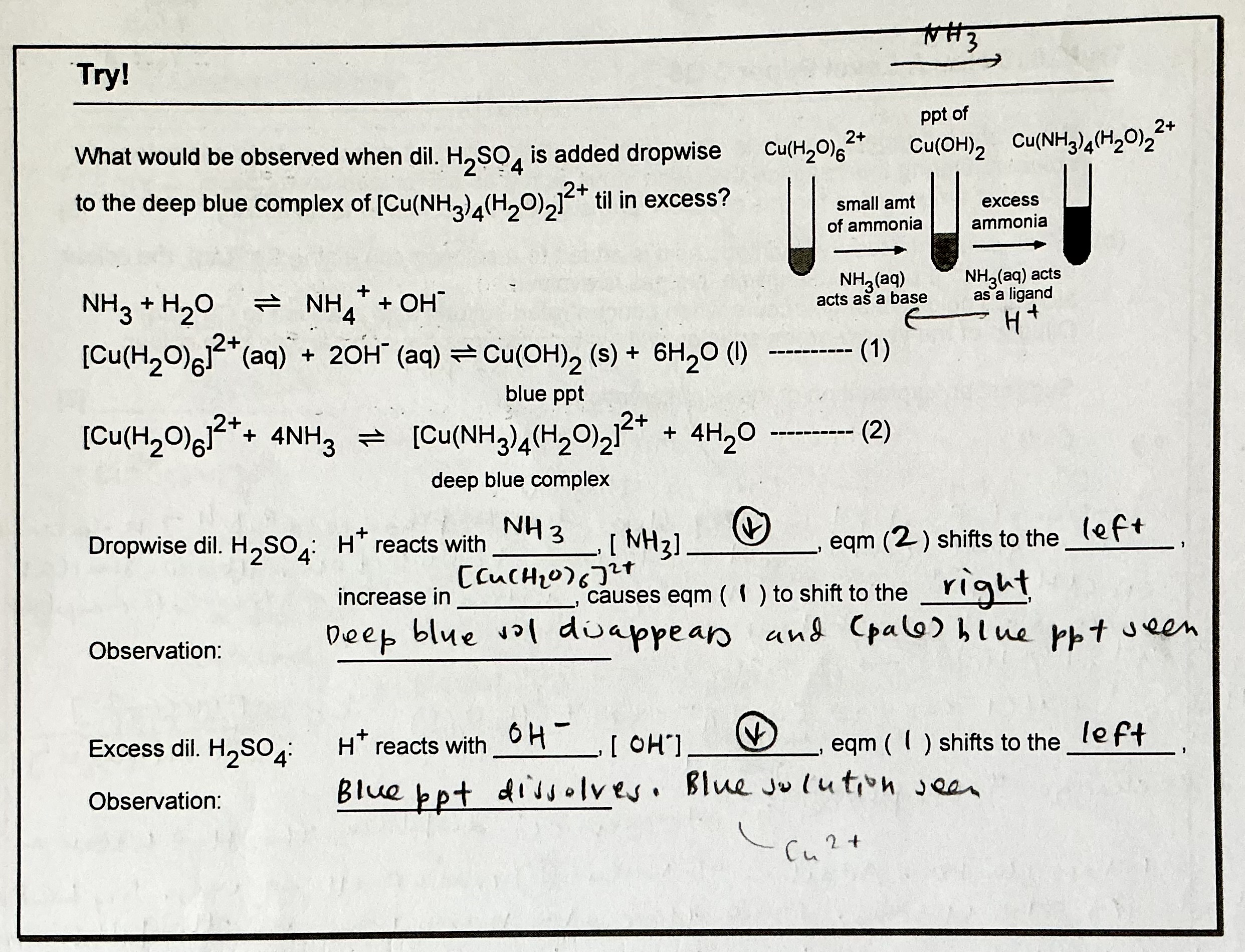
Explain the observation when NH3 is added to Cu²+, and write any relevant equations.
Explain the observation when dilute H2SO4 is added to [Cu(NH3)4(H2O)2]2+ dropwise, till in excess. Write any relevant equations.
Why is it dangerous for CO or CN- to bind to haemoglobin? What symptoms may one display?
Carbon monoxide replaces weaker H2O ligands in haemoglobin, by binding irreversibly. This prevents formation of oxy-haemoglobin and hence cells are deprived of oxygen. This accounts for toxic nature of CO.
→ Small amount of carboxyhaemoglobin → may cause tiredness, dizziness, unconsciousness
→ Large amount of carboxyhaemoglobin → may cause carbon monoxide poisoning which can lead to suffocation and death
Why may transition metals and their ions make effective heterogenous catalysts? Why may transition metals and their ions make effective homogenous catalysts?
→ HETERO: They have partially filled 3d orbitals which allow the reactant particles to be adsorbed onto the catalyst surface.
→ HOMO: Transition metal ions can exist in different oxidation states and can undergo conversion from one oxidation state to another relatively easily.
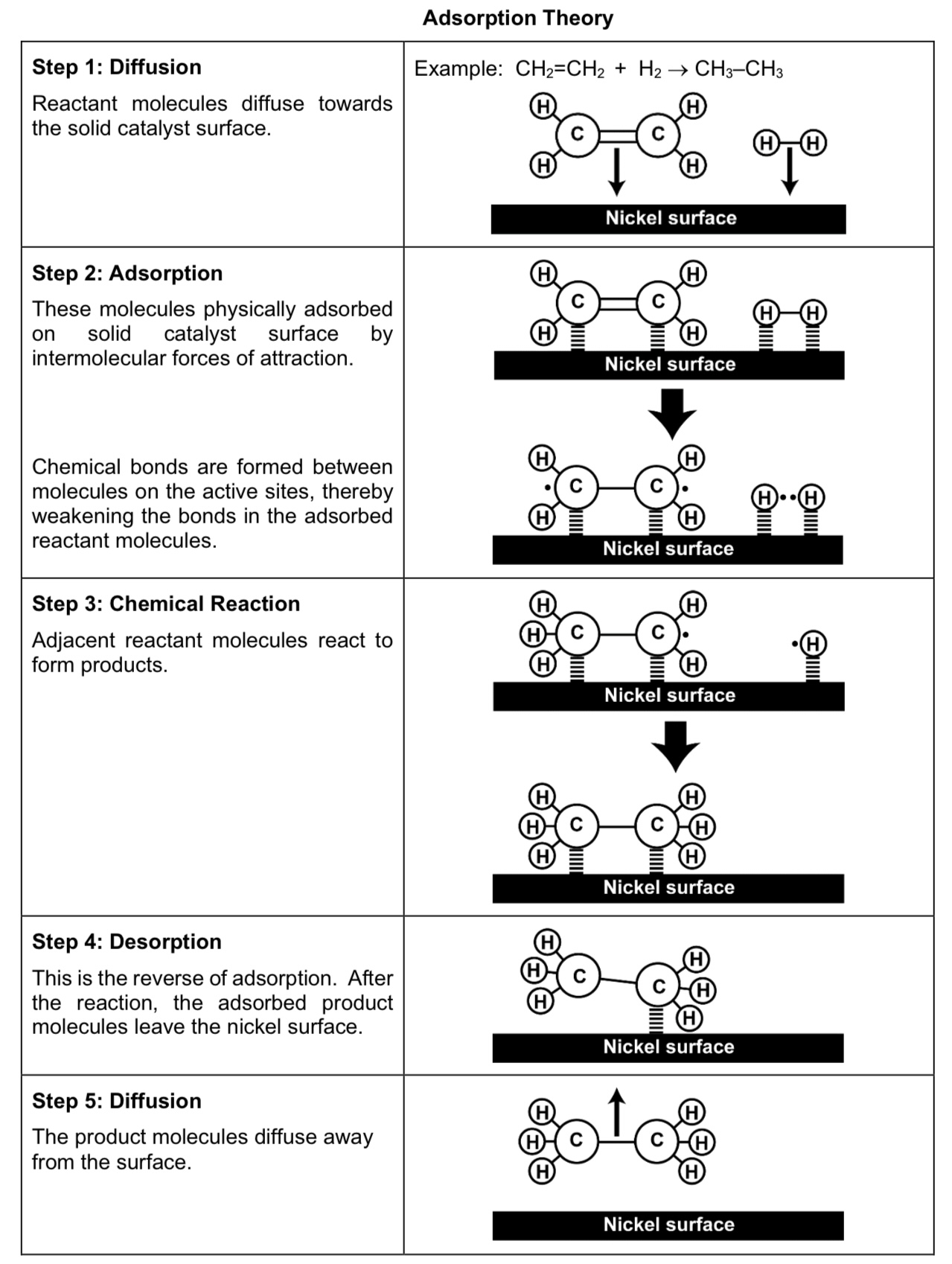
Outline the mode of action of heterogenous catalysts.
1) Reactant molecules diffuse towards solid catalyst surface.
2) The molecules physically adsorb onto solid catalyst surface by IMF. Chemical bonds are formed between molecules on active sites, which weakens the intramolecular bonds in the adsorbed reactant molecules.
adsorption weakens covalent bonds within reactant molecules
adsorption increases conc of reactant molecules
3) Adjacent reactant molecules react to form products.
4) After the reaction, the adsorbed product modules leave the catalyst surface.
5) The product molecules diffuse away from the catalyst surface.
Why do catalysts speed up reactions?
They increase the rate of reactions by providing an alternative pathway of lower activation energy for the reaction to occur.