1.10-1.13 Separating Mixtures
1/20
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What is distillation?
separates solute and solvent in solution
What is fractional distillation?
A process used to separate mixtures of miscible substances with different boiling points
Miscible
describes liquids that dissolve in one another in all proportions
What is Filtration?
the process that separates a solid from the liquid in a mixture
What is crystallisation?
The formation of crystals by cooling a saturated solution
What is a separating funnel?
Separates immiscible liquids
What is paper chromatography?
an analytical method used to separate colored chemicals or substances
Method of colour chromatography
Set up chromatography paper with a horizontal pencil line at the bottom with ink spots to be tested
Lower paper into a beaker with appropriate solvent. Wait for solvent to travel up
Analyse
What does it mean if the ink does not travel up the chromatography paper?
It is insoluble in the solvent
How do you find the Rf value?
Distance travelled by the component / distance travelled by the solvent front
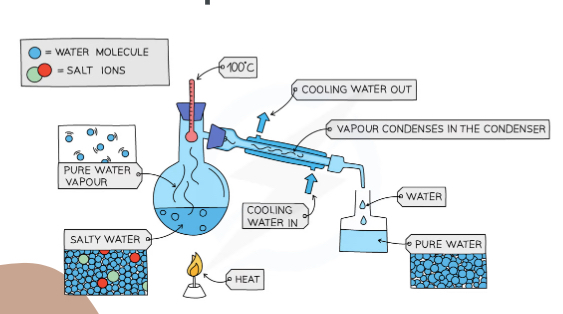
What separation technique is this?
Simple distillation
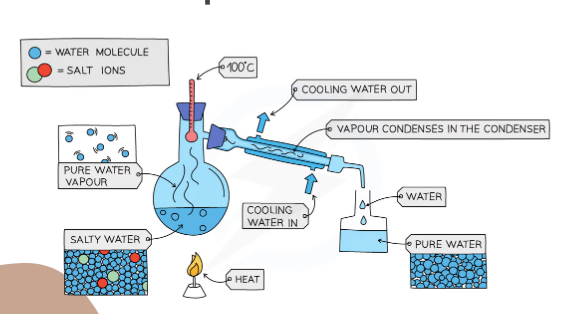
Explain what is happening in this image
The water salt solution is boiling at 100C and the water turns into gas and then is condensed back into liquid by the condense and the salt stays in the flask
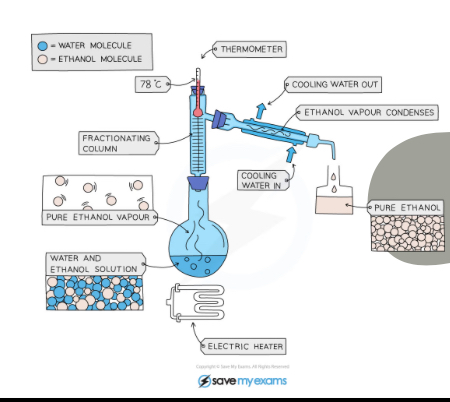
What separation technique is this?
Fractional distillation
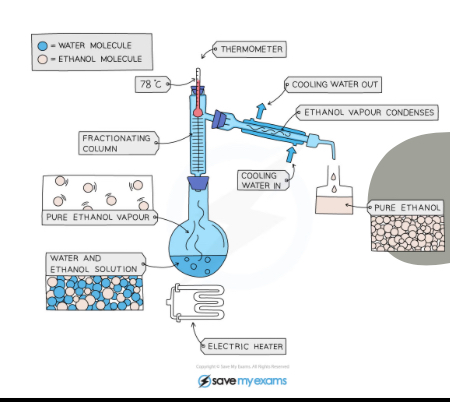
Explain what is happening in this picture
The water 100C and ethanol 78C have different boiling points so the ethanol water solution is boiled at 78C and the ethanol is boiled into gas and condenses in the condenser and the water stays in the flask
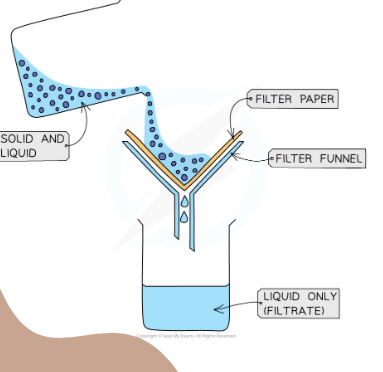
What separation technique is this
Filtration
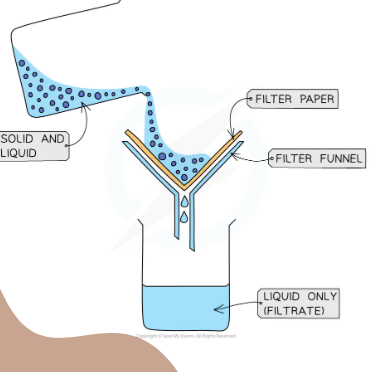
Explain what is happening
The solid and liquid is poured into a funnel with filter paper which catches all of the solid (residue) and the liquid filtrates through into the flask (filtrate)
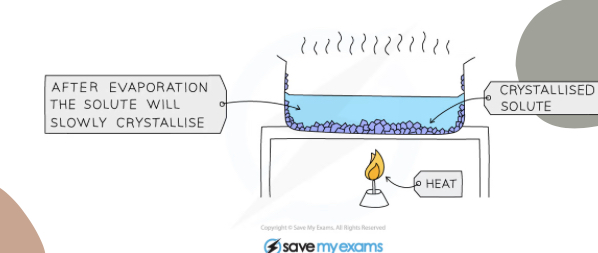
What separation technique is this
Crystallisation
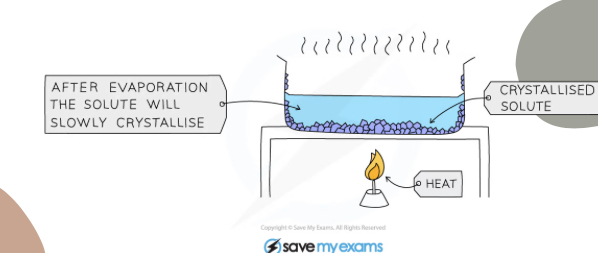
Explain what is happening
The solution is heated until almost saturated, heat is turned off and crystals form as water evaporates and solution cools, then it can be separated by filtration
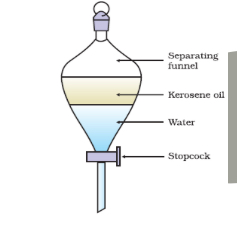
What separation technique is this
Separating tunnel
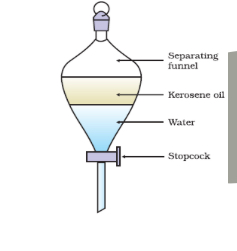
Explain what is happening
The mixture is placed inside the separating funnel to settle. The less dense liquid will form a layer above the more dense layer below. The tap can be used to remove the more dense bottom layer.
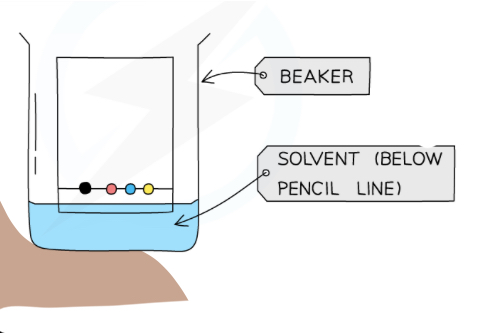
Name this separation technique
Paper chromatography