Chapter 2: Atoms, Molecules, and Ions
The Atom
Atoms are mostly empty space.
Subatomic Particles
Protons have a positive charge.
Neutrons have a neutral charge.
Electrons have a negative charge.
Protons and neutrons are found in the nucleus and essentially have the same mass.
Electrons travel around the nucleus.
Atomic Number
Atomic number: the number of protons in the nucleus of an atom; it also provides the number of electrons an atom has.
The number of protons equals the number of electrons in an atom.
Atoms of an Element
Elements are represented by a one or two letter symbol.
All atoms of the same element have the same number of protons, which is the atomic number. It is the subscript before the symbol.
The mass number is the total number of protons and neutrons in the nucleus of an atom. It is the superscript before the symbol.
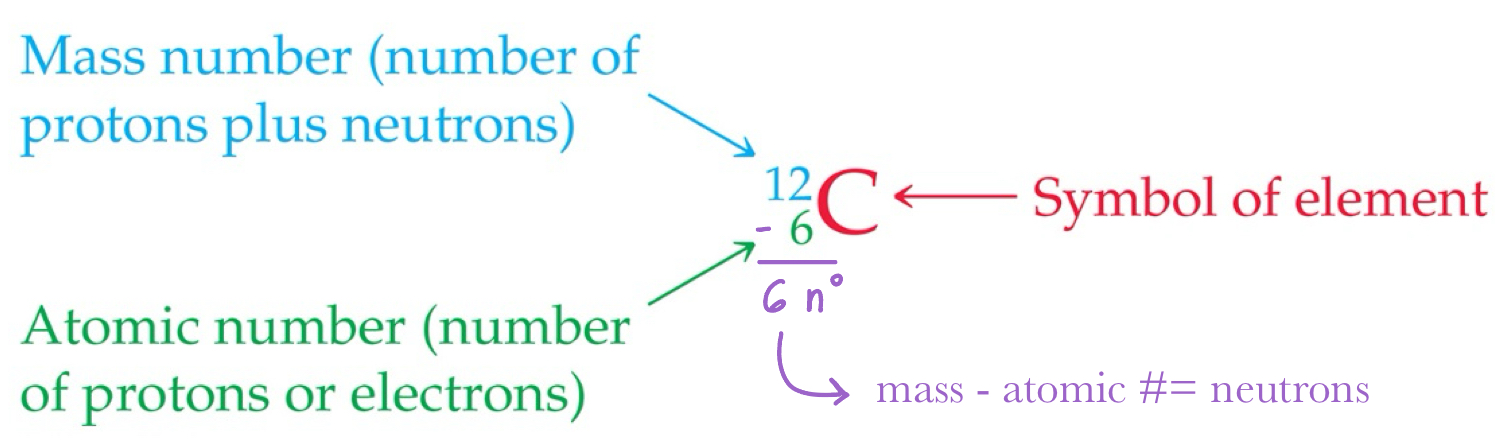
Isotopes
Isotopes are atoms of the same element with different masses.
Isotopes have different numbers of neutrons, but the same number of protons.
Example: Isotopes of Carbon
Symbol | Number of Protons | Number of Electrons | Number of Neutrons |
11C | 6 | 6 | 5 |
12C | 6 | 6 | 6 |
13C | 6 | 6 | 7 |
14C | 6 | 6 | 8 |
Atomic Mass Unit (amu)
Atoms have extremely small masses.
A mass scale on the atomic level is used, where an atomic mass unit (amu) is the base unit.
1 amu = 1.66054 × 1024- g
Atomic Weight
Atomic weight: an average mass is found using all isotopes of an element weighted by their relative abundances.
Atomic Weight Formula:
\sum [(isotope mass)x(fractional natural abundance)] for ALL isotopes (add same equation for all isotopes).
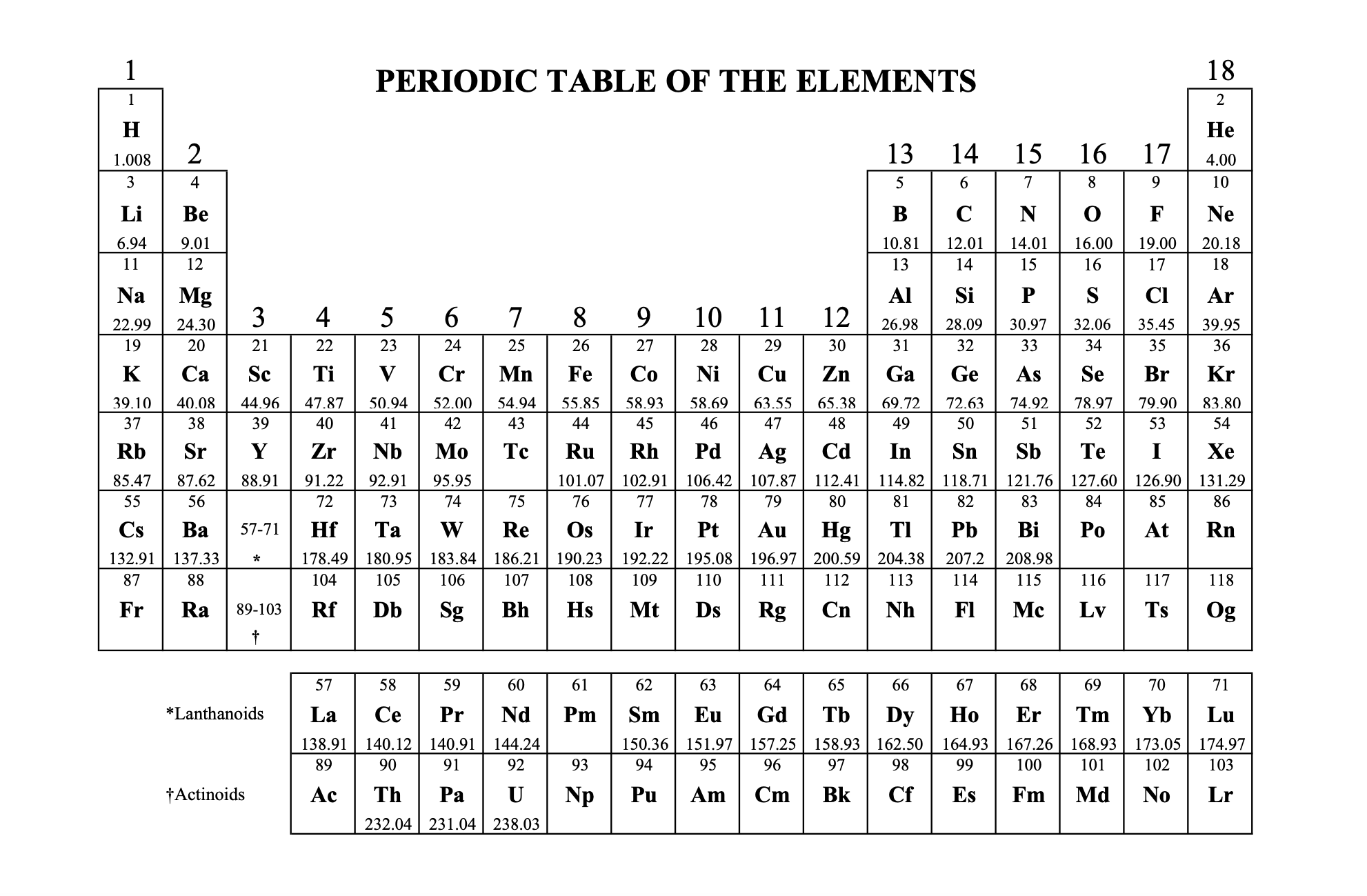
The Periodic Table
The periodic table is a systematic organization of the elements.
Elements are arranged in order of atomic number.
Organized into groups and periods.
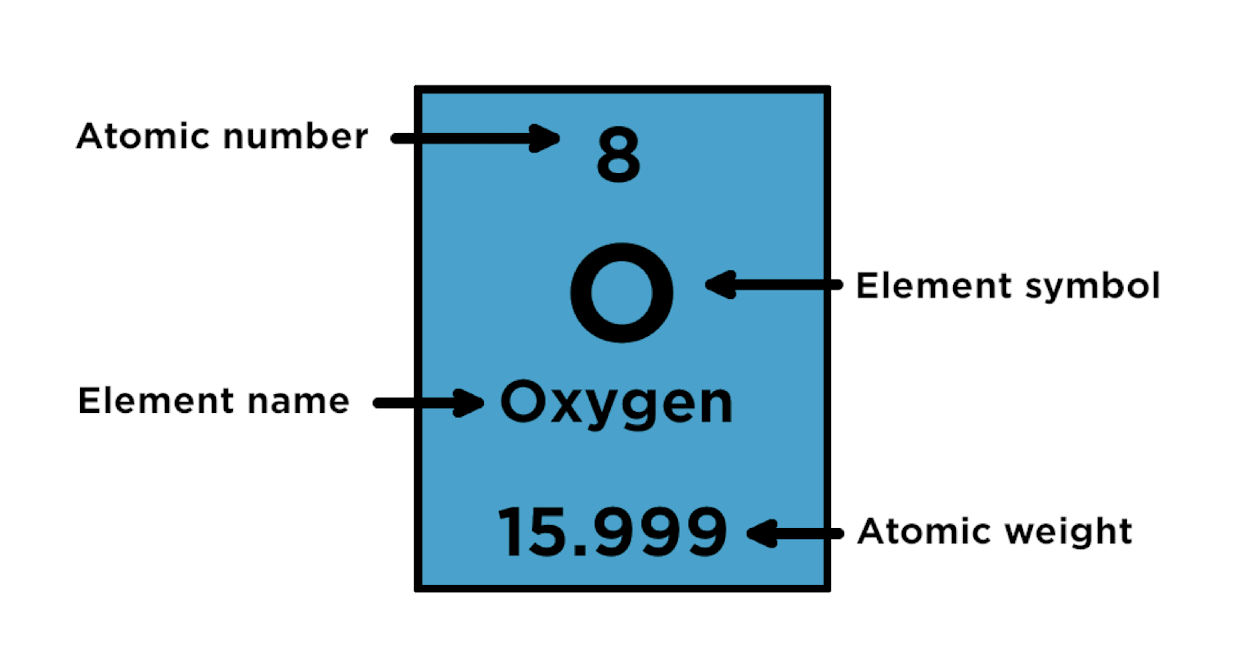
Reading the Periodic Table
The atomic number is above the symbol.
The atomic weight is below the symbol.
Atomic number = amount of protons and electrons.
Atomic weight = protons + neutrons
Organization of the Periodic Table
The rows on the periodic table are called periods.
Columns are called groups.
Elements in the same groups have similar chemical properties.
Group Names
1A are Alkali metals (Li, Na, K, Rb, Cs, Fr)
2A are Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra)
3-12 are Transition metals
6A are Chalcogens (O, S, Se, Te, Po)
7A are Halogens (F, Cl, Br, I, At)
8A are Noble gases (He, Ne, Ar, Kr, Xe, Rn)
Metals, Nonmetals, and Metalloids
Metals are on the left side of the periodic table.
Properties include shiny luster, conducting heat and electricity, and solids (except Hg).
Nonmetals are on the right side of the periodic table (they include Hydrogen, H).
They are mostly gases, but can be solid (Carbon), liquid (Bromine), or gas (Neon) at room temperature.
Metalloids occur at a “stair step”.
They include Boron, Silicon, Germanium, Arsenic, Antimony, and Tellurium.
Their properties are sometimes like metals and sometimes like nonmetals.
Chemical Formulas
The subscript to the right of the symbol of an element tells the number of atoms of that element in one molecule of the compound.
Molecular compounds are composed of molecules and almost always contain only nonmetals.
Diatomic Molecules
These seven elements occur naturally as molecules containing two atoms:
Hydrogen (H2)
Nitrogen (N2)
Oxygen (O2)
Fluorine (F2)
Chlorine (Cl2)
Bromine (B2)
Iodine (I2)
They can never exist alone, they must be paired with something else.
Types of Formulas
Empirical formulas give the lowest whole-number ratio of atoms of each element in a compound; simplest formula.
Molecular formulas give the exact number of atoms of each element in a compound; actual formula.
If we know the molecular formula of a compound, we can create its empirical formula.
Ions
When an atom of a group of atoms loses or gains electrons, it becomes an ion.
Ion - a charged atom.
Cations are formed when at least one electron is lost.
Monoatomic cations are formed by metals.
Group 1A (1+) and 2A (+2)
Trick: Cation = paw-sitive
Anions are formed when at least one electron is gained.
Monoatomic anions are formed by nonmetals, except the noble gases.
Groups 5A (3-), 6A (2-), and 7A (1-)
Polyatomic Ions
Sometimes a group of atoms will gain or lose electrons.
Polyatomic ions - many atoms grouped together.
Polyatomic cation example: Ammonium is NH_{4}^{+}
Polyatomic anion example: Sulfate is SO_{4}^{2-}
Ionic Compounds
Ionic compounds (such as NaCl) are generally formed between metals and nonmetals.
Metal + Nonmetal
Cation written first then anion.
Electrons are transferred from the metal to the nonmetal. The oppositely charged ions attract each other. Only empirical formulas are written.
Writing Formulas
When writing formulas, the charge of the cation becomes the subscript for the anion.
The charge for the anion becomes the subscript for the cation.
If they are not the lowest whole-number ratio, divide them by the greatest common factor.
Chemical Nomenclature
The system of naming compounds is called chemical nomenclature.
Inorganic nomenclature
Write the name of the cation. If the cation can have more than one possible charge, write the charge as a Roman numeral in parentheses. If it is a polyatomic cation, it will end in -ium.
If the anion is an element, change its ending to -ide; if the anion is a polyatomic ion, simply write the name of the polyatomic ion.
Nomenclature of Binary Molecular Compounds
The name of the element farther to the left in the periodic table or lower in the same group is usually written first.
A prefix is used to denote the number of atoms of each element in the compound.
The prefix mono is not used on the first element listed.
Prefixes:
Prefix
Meaning
mono-
1
di-
2
tri-
3
tetra-
4
penta-
5
hexa-
6
hepta-
7
octa-
8
nona-
9
deca-
10
The ending of the second element is changed to -ide.
CO2: Carbon dioxide
CCl4: Carbon tetrachloride
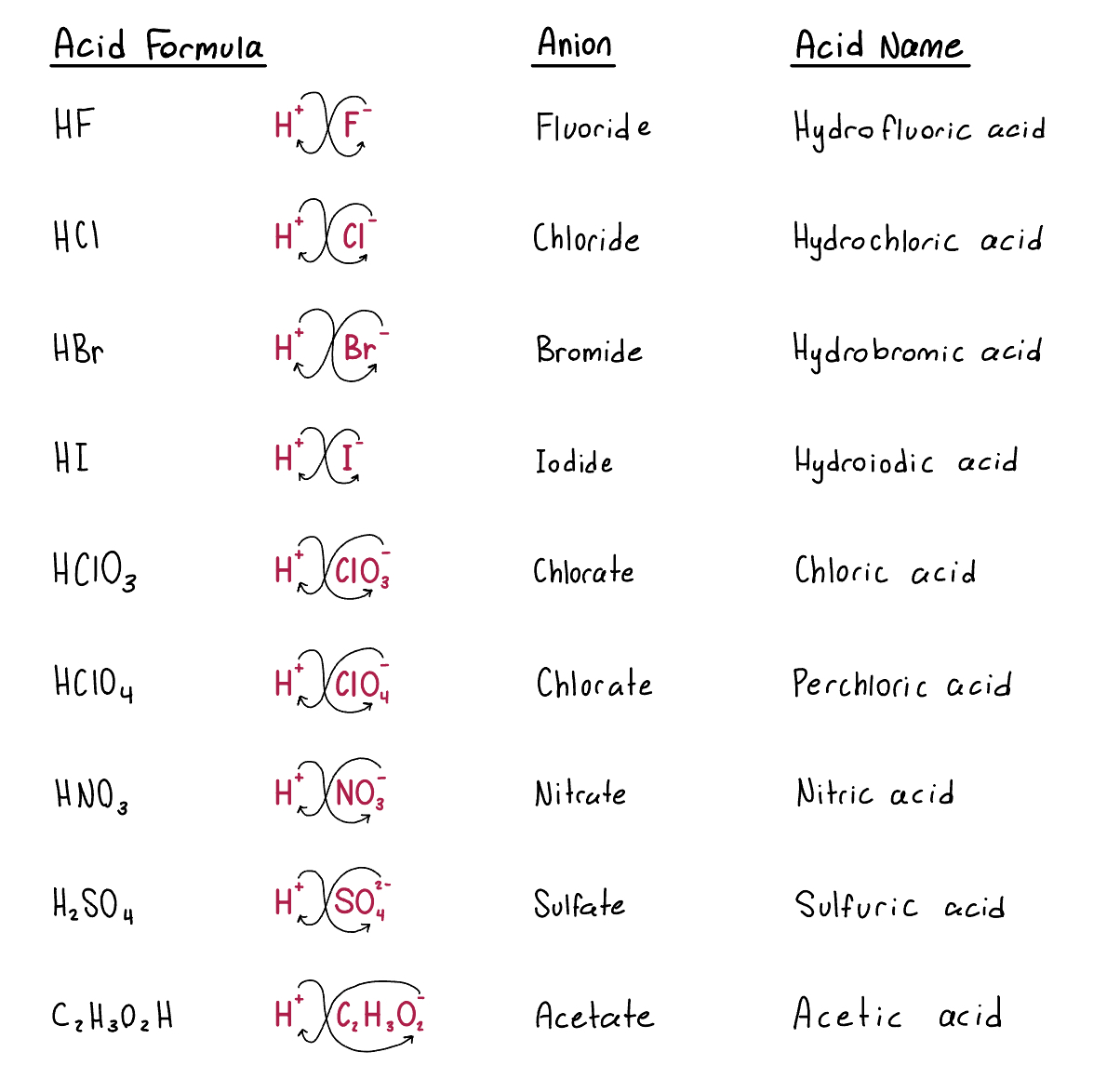
Acid Nomenclature
Three Rules for Acid Nomenclature
If the anion in the acid ends in -ide, change the ending to -ic acid and add the prefix hydro-.
HCl: hydrochloric acid
HBr: hydrobromic acid
HI: hydroiodic acid
If the anion ends in -ite, change the ending to -ous acid.
HClO: hypochlorous acid
HClO2: chlorous acid
If the anion ends in -ate, change the ending to -ic acid.
HClO3: chloric acid
HClO4: perchloric acid
Helpful mnemonic device for naming acids:
My ride has hydrolics (-ide changes to hydro____ic acid)
I ate something icky (-ate changes to -ic acid)
Sprite is delicious (-ite changes to -ous acid)
Reference: Chemistry The Central Science (14th Edition)