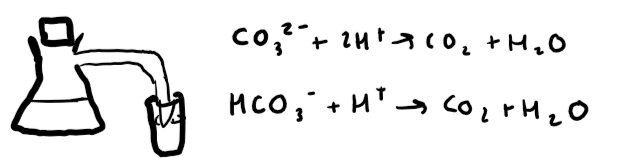Topic 4: Inorganic tests
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
How do you test for metals?
Dip nichrome inoculating loop in concentrated hydrochloric acid.
Place loop into the blue Bunsen flame to remove any impurities.
Dip the nichrome inoculating loop in concentrated hydrochloric acid again.
Then dip the loop into your sample.
Next, place the loop into the blue Bunsen flame and observe the colour.
Why do we use a nichrome inoculating loop?
Inert + has a high melting point
Why do we use HCl?
It will produce a volatile chloride salt.
Why do we see different colours in the metal flame test?
Energy absorbed from the flame excites electrons and causes them to move to higher energy levels. The colours are then seen as the electrons fall back into lower energy levels, releasing/emitting energy in the form of light. The difference in energy between the higher and lower levels determines the wavelength of light released- which determines the colour of the light.
What are positive results for the following metals in a flame test: lithium, sodium, potassium, rubidium, caesium, calcium, strontium and barium?

How do you test for halide ions?
Silver Nitrate test
Add dilute nitric acid (HNO3) and silver nitrate solution (AgNO3) to unknown halide ion. + water bath
Why is nitric acid used in a silver nitrate test for halide ions and why are other acids not suitable?
Nitric acid, as NO3- is soluble. Reacts with carbonate ions (CO3-) which could leave to a false positive.
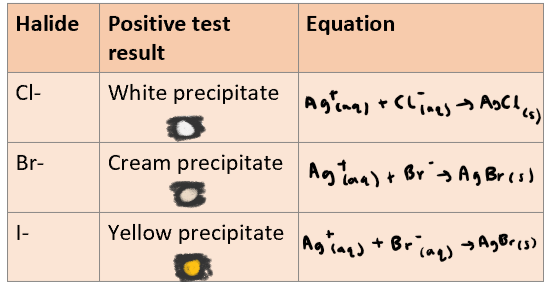
What do you see in a Silver Nitrate test? Include observations + reaction equations
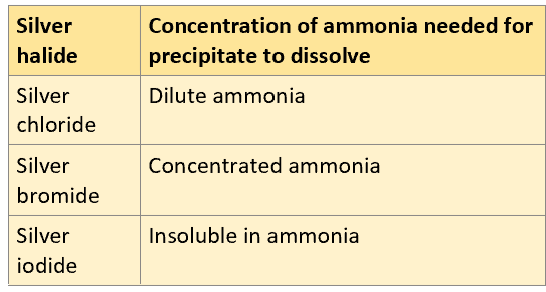
How much ammonia is needed to dissolve each silver halide?
How do you test for ammonium compounds? + reaction
Add sodium hydroxide, gently heat and if ammonium compound present, ammonia gas will be produced. Test for this using a damp red litmus paper.
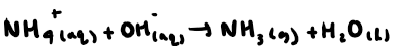
What do you see in a positive test for ammonium compounds?

How do you test for hydroxides?
Use damp red litmus paper
What is a positive result for hydroxides?
Red damp litmus paper turns blue
How do you test for sulphates? + reaction
Add hydrochloric acid to remove any carbonates in it (these could precipitate out after barium chloride giving a false result). Then add barium chloride.
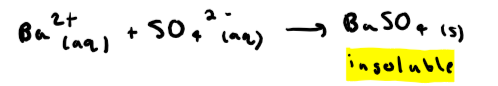
What do you see in a positive test for sulphates?
White precipitate
How do you test for carbonates? + include positive result and reaction
Add hydrochloric acid to carbonate to make CO2 gas. Bubble through limewater.