Oxygen Physiology Midterm set
1/166
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
167 Terms
What is primordial nucleosynthesis?
100 seconds after the Big Bang
Deuterium --> 4-He (3:1 ratio of 1-H to 4-He)
In the first 100 seconds of the universe... H, He and trace amounts of Li and Be
What is the stability gap?
Occurs when Neutrons + Protons = 8
Cannot jump past 7-Li to get to 8-Be; it is too unstable to add a proton and a neutron.
There is a similar gap from 4-He to 6-Li
How many years ago can the origin of oxygen in the universe be dated to?
14 billion years ago
How did we get oxygen if we were limited by initial 4 elements? (What were those 4 elements?)
First 4: H, He, trace Li and Be
Stellar nucleosynthesis brought about C and O 14 billion years ago.
2 4-He atoms fused to become a 8-Be (which is very unstable) and then an 8-Be fused with another 4-He to become 12-C.
Then 12-C fused with a 4-He to become a 14-O.
What is the stability endpoint?
56-Fe
Anything undergoing fission will fission down to 56-Fe
Anything undergoing fusion will fuse up to 56-Fe
What is the origin of O2 on the Earth dated to?
4.5 billion years ago
What are the periods to note and their associated milestones?
Hadean - 4.5 bya lava, brimstone, fire
Archaen 4 bya - Sulfur isotopes (bacterial life began)
Proterozoic 2.5 bya - Multicellular organisms
Paleozoic 550 mya - Vertebrates, first land plants, Cambrian explosion
Mesozoic 250 mya - Dinosaurs (Triassic, Jurassic, Cretaceous)
Cenozoic 65 mya - Mammals
The first atmosphere included
hydrogen and He
What was the classical primordial atmosphere makeup?
A reducing atmosphere! (<1 ppm O2)
H2, NH3, CH4, H2O and H2S
All reduced H in these gases due to the atmosphere
What is the importance of the intial reducing conditions of earth?
- Organic molecules could not accumulate in the presence in presence of oxygen
- Photosynthesis develops using H2S
- Radical defense develops against sulfur radicals.
- First life in sea vents; oxygen in the ocean would've disrupted it
What are volcanic gases comprised of?
50-60% water vapor
24% CO2
13% Sulfur
6% Nitrogen
Trace gases Ar, He, Ne, CH4, CO, H2
When is the origin of life on Earth dated?
3.5 bya
When was the "great oxidation event" and what likely induced it?
~2.3 bya
Induced by cyanobacteria through photosyntehsis. Before the GOE, any free O2 they produced was chemically captured by dissolved iron or organic matter. The GOE was the point in time when these oxygen sinks became saturated at which point oxygen, produced by cyanobacteria was free to escape into the atmosphere.
A cooling Earth meant less volcanic reducing gases to titrate the O2 from cyanobacteria
What is significant about banded iron formations?
Give insight into the change of Earth's atmosphere over the course of time (from reducing to oxidizing)
What is the "snowball Earth"?
Proposed by Joseph Kirschvink - 1992
Approx 650 mya (Proterozoic era) Earth's surface was nearly entirely frozen.
Facilitated by equatorial continent distribution
Volcanic activity releases greenhouse gases mainly CO2 which begins the global warming cycle
Glaciation reduces photosynthesis and thus O2 production
Oxygen accumulation in the atmosphere came from what reaction?
- CO2 + H2O = CH2O + O2
Carbon dioxide plus water yields formaldehyde and oxygen gas.
Excess oxygen comes from loss of formaldehyde by burial of organic matter in earth crust
What is pyrite?
Fools gold (iron sulfide)
What is the significance of the long-term global cycles of carbon and sulfur?
Because little carbon can be stored in the ocean, inequalities between the weathering and burial of organic matter results in reciprocal formation or loss of calcium carbonate to preserve carbon. The same is true for iron sulfide, calcium sulfate, and suflur. Any prolonged imbalance in the net flux between oxidized and reduced reservoirs of carbon must be balanced by opposite fluxes between reduced and oxidized reservoirs in sulfur to avoid fluctuations in O2 to maintain life over time
What did Mike Newman discover?
Millipede like creature that turned out to be 420 million years old with microscopic airholes (a fossil)
Pneumodesmus newmani
"Fermentation is life without oxygen" Fermentation of organic compounds in the absence of oxygen. Substrate level oxidation
Attributed to?
Louis Pasteur
"Atavistic genes of modern organisms often betray their evolutionary roots"
Nick Lane 2002
What is the energy equation for life?
2H2 + O2 = 2H2O + Energy
Hydrogen is the fuel here.
Life requires energy. Life is separation from the energy state of the universe. Life is a state of dynamic disequilibrium.
What is the prevailing view of atmospheric oxygen evolution over time?
That proxies set an upper limit for oxygen levels before 2.45 bya and a lower limit after that time.
The record of oxidative weathering after 2.45 bya ago sets a lower limit for oxygen levels at 1% of present atmospheric level and and upper limit of 40% (inferred from the evidence of the anoxic oceans during the Proterozoic). There are tighter bounds on O2 from 420 mya to the present which is set by a continuous record of charcoal accumulation: flames cannot be set below an oxygen level of 60% PAL and above 160% of PAL.
What does the oxygen level need to be for flames to render (in regards to PAL)?
Needs to be at least 60% PAL and can't be more than 160% PAL.
What is the current atmosphere components?
Dry gas oxidizing atmosphere
20% O2
79% N2
0.03% CO2
Trace noble gases
What happens during the Precambrian era?
beginning of life, photropic bacteria, cyanobacteria plus phototrophs, macroscopic eukaryotes, and the beginnings of algae and shelly invertebrates
It covers 90% of the history of the Earth up until 600 mya.
After that comes the Paleozoic, the Mesozoic, and the Cenozoic (current era)
When were the first real fossils found?
At the boundary of the Cambrian era (600 mya); around the proterozoic era.
What facilitated warm temperatures?
Consider global warming!
Water and CO2 in the atmosphere allowed warmer temperatures (15C vs -18 C)
Predation favors
- growth in size of both predator and prey
- Oxidation is about 40% efficeint so there can be 6 levels where predators can still survive
Substrate level oxidation is __% efficient
10%
What effect did atmospheric oxygen have on body size and mass?
Exhibited in Drosophila melanogaster
Greater oxygen saturation and tolerance: larger body size and mass
What was Galen? What words were ascribed to him?
Physician to Marcus Aurelius
"Bios" - duration of life
"Zoe" - ensemble of actions; "zoon" - living being, process of being
"Psyche" - soul: confers the ability to move for the being
"Thymos" - life = spirit
What is the quintessential physiology diagram?
It was a diagram used in Galen's time that reduced the world down to irreducible elements and had biological components ascribed to them:
Earth (black bile)
Wind (blood)
Water (phlegm)
Fire (yellow bile)
This system worked for a 1000 years to classify people and their illnesses
Who was Erasistratus?
pneuma zotikon - vital spirit arterial
pneuma psychikon - animal spirit nerves
Breath
- Anemos - soul
- Pneuma - air, breath of life
- Psyche -spirit mind
What did Davinci contribute?
b. 1452
Air is is composed of at least two components.
"Air which does not support fire, does not support life"
What did William Harvey contribute?
Figured out that blood only moves in one direction (showed that there were valves in the venous circulation)
What did Malpighi contribute?
Proved Harvey's hypothesis by modeling it on a frog (gave Harvey's concept a mechanism)
What did Evangelista Torricelli contribute?
b. 1600s
Invented the mercury barometer and demonstrated the existence of a vacuum (although did not publish it because it was scientific heresy; went against Aristotle's findings that were supported by the Church)
What did Pascal contribute?
Substantiated Torricelli's ideas of a vacuum and showed that air has weight that is diminished by height.
What did Dalton and Boyle contribute?
- Dalton's law of partial pressures
- Boyle showed that in a partial vacuum animals die and that flames go out
What did Joseph Black contribute?
Discovered CO2 and called it "fixed air" by pouring acid on chalk and observing the bubbles (the gas in the bubbles was heavier than air)
also constructed an accurate pan balance
What did Henry Cavendish contribute?
- Discovered Hydrogen and called it phlogistin.
- said that water was 2 parts hydrogen and one part dephlogisticated air (oxygen) which proved that water was not an essential element because it could be broken down into parts
-Figured out air was 20% oxygen
What did Rutherford do?
He was Cavendish's student that discovered Nitrogen (N2) and called it "noxious air" or phlogisticated air
What is phlogistin? When was the theory prominent?
1667-1774
When you burn something, it weighs more because you're adding something (we know now that it's oxygen)
But they thought it was because of the fire principle that was being taken away and termed them phlogistin reactions.
So phlogisticated air is when you take oxygen out of the air. Essentially, the air is so saturated with "phlogistin" that you can't light a fire.
Who theoretically discovered oxygen then?
Priestley* b. 1733-1804
Lavoisier*
What did Joseph Priestley contribute?
- Worked for a brewery and if you scooped the air over the beer and passed it into the water you could get something refreshing - he invented carbonated water (also known as Black's gas, or fixed air, into water) which was proposed as a cure for scurvy
- Discovered HCl
- Discovered O2 via a nitric oxide assay; he generated O2 by burning mercuric ocide
- Discovered CO when he moved to Pennsylvania
What did Carl Scheele contribute?
Concurrently discovered oxygen in Sweden but was slow to publish
Called it "fire air" (a more appropriate name)
What did Antoine Lavosier contribute?
"Discovered" oxygen using Scheele's and Priestley's assertions...
- Worked for the French Army producing gunpowder, collected taxes, became nobility and was later executed in the French Revolution
- Wrote a treatise on oxygen without crediting Priestley
- Named "oxygen" because he though oxygen is what made acid an acid (literally "acid generator").
- Connected oxygen with burning (like Scheele)
- Replaced the phologistin theory with Joseph Black's caloric theory (heat consists of a self-repellant fluid that flows from hotter to colder bodies)
What were the eight sages that contributed to the discovery of oxygen?
Priestley
Lavoisier
Scheele
Cavendish
Ibn al-Nafis
Servetus
Sendivogius
Mayow
What did Ibn al Nafis do?
Described the pulmonary circulation
What did Servetus do?
Described blood flowing through the lungs and how it changed color doing so (oxygenated vs. deoxygenated blood)
What did Sendivogius do?
Air contains the "Secret Food of Life" - gas emitted by potassium nitrate when heated
What did Mayow do?
Wrote about air he names SPIRITUS NITRO-AEREUS. He said it is consumed in the fire and that our body utilizes it to provide body heat and energy.
Who was Edward Morley?
- A professor here at Western Reserve;
- Worked in the Sanitary Commmision during the Civil War where many soldiers died of dysentery. Also did forensic science as a side job as well as maintained a clock in Cleveland.
- STUDIED THE CONCENTRATION OF O2 in various spots and altitudes around the world and came up with the Morley-Loomis hypothesis that states O2 % is constant everywhere (21%)
- Found the atomic weight of O2 and 15.879 which worked to refute Prout's hypothesis that atomic weights were only whole number integers of hydrogen
What are the most important isotopes of oxygen? Which are stable and not stable?
STABLE
16-O (99.76%)
17-O (0.04%)
18-O (0.2%)
NOT STABLE
15-O (used as a positron emitter e.g. PET scans and in metabolic experiments)
What are some uses of the isotopes of oxygen?
- 16-O and 18-O have changed over time with temperature so measuring the concentrations of these as a ratio we can extrapolate temperatures in different time periods
15-O is a positron emitter that is used in PET scans and in metabolic experiments
17-O has a large NMR singlet (5:2 nuclear spin, whereas the other isotopes do not have spins)
What is the Cunningham Steel Ball?
It was built in Cleveland and was used for hyperbaric oxygen therapy. After the AMA declared such therapy was quackery, it was dismantled and used in military vehicles.
What are the primary pathologies associated with hyperoxia and where do they occur?
Lorrain-Smith effect:
Lung damage due to oxygen intoxication
Paul Bert effect:
Acute CNS symptom where among patients placed in a hyperbaric oxygen the first problem that was induced was seizures
John Bean effect:
Chronic spinal paralysis (at pO2s that weren't quite enough to cause the Lorrain-Smith effect, paralysis could occur)
2 of the 3 pathologies associated with hyperoxia affect the nervous system
3 ppm O2 = 1 RAD
What are the different states of oxygen in terms of orbitals?
Ground state triplet diatomic oxygen has 2 unpaired electrons with parallel spin in the 2p pi* orbitals -- is a diradical
Singlet delta O2 (ground singlet state) has one set of paired electrons in the 2p pi* orbital
Singlet sigma O2 (excited singlet state has two unpaired electrons with opposite nuclear spin (shortest half life; highly reactive) -- is a radical
What is the first reduction state of diatomic oxygen?
Superxoide anion
Oxygen →+ 1 e-→ Superoxide anion radical →+ 1 e-→ Peroxide → + 1 e-, + H+ → Hydroxyl radical → +1 e-, +H+ → H2O
Are radicals inherently more dangerous?
Not necessarily.
Example, ground state triplet oxygen has two unpaired electrons while peroxide does not have unpaired electrons yet it is more reactive than the diradical ground state triplet diatomic oxygen.
What is a radical?
A molecule with an unpaired valence electron (unpaired electron in outermost shell)
What is the Fenton reaction? What does it show?
Fe2+ H2O2 → Fe3+ + OH* + OH-
Fe3+ + H2O2 → Fe2+ + HOO* + H+
Free iron is dangerous as is seen in hemolysis (breakdown of hemoglobin)
Haber-Weiss elaborates on the Fenton reaction → wherever you have free iron, there is radical production which can be buffered by proteins.
What are the defenses the body has against ROS?
1. Avoidance - oxygen is kept away from processes and organs unless it's necessary (blood flow control)
2. Safe use - e.g. cytochrome oxidase (binds oxygen and holds it in a pocket until 4 e- are transferred and then water is released)
3. Enzymatic -
a. superoxide dismutase/catalase
b. glutathione peroxidase (reduces H2O2 by transferring electrons from peroxide to glutathione; the amount of oxidized glutathione, GS-GS, can be measured against reduced glutathione to get a measure of oxidative stress)
4. Quenching -
a. Vit E - (fat-soluble, takes longer to work) grabs the electron from the radical
b. Vit C - regenerates Vit E (water-soluble, works immediately)
c. beta-carotene
What is lipid peroxidation in the context of ROS?
Has to do with how quenching agents like Vit E and Vit C can protect AGAINST lipid peroxidation which is explained thusly:
If a radical attacks double bonds in a lipid it can produce a molecular rearrangement and interact with the next lipid in the chain causing a propagation of rearrangements and ultimate destruction of the membrane fluidity and ultimately kill the cell.
What's an example of radical damage in fruit, for example?
Polyphenol oxidase is an enzyme in fruit.
Takes phenols in fruit and reacts them with oxygen to produce melanin.
Can be warded off by rubbing lemon juice on apple because lemon juice contains ascorbate (Vit C) which can accept that extra electron without turning into a radical itself.
What are some quantitative methods of measuring oxygen?
1. Polarography → graphing current against voltage where current coming of the circuit is related to the amount of oxygen in a voltage-current relationship.
This was done by LaManna where an electrode was inserted into a mouse brain where they found that, in resting states, oxygen values were low (method of ROS defense; if the brain needs oxygen circulation will increase transiently and then go away again).
2. Optical methods
a. Fluoroscence quenching → oxygen interferes with fluorophores; can be quantified via the Stern-Volmer equation:
I(o)/I = 1 + K(sv)[Q]
b. Hemoglobin absorption (blood gas) → better for higher pO2 values
Myoglobin absorption → better indicator between 2-10 Torr
c. Luminescence and phosphorescence → same idea as fluorescence but the photon response is delayed and this delay is oxygen-sensitive.
d. Electron Paramagnetic Resonance (EPR) → oxygen changes the line width where a more narrow line indicates less oxygen and wider indicates more oxygen. Oxygen tension EPR measurement can be focused with a crystal or with metal dyes (tattoos).
What are some qualitative methods for measuring O2 concentrations?
1. Mass spec → not good for short-term changes
2. NMR/MRI (17-O) - breathe in oxygen with 17-O and then measure the rate at which water vapor labeled with 17-O appears.
3. PET (positron emitter, 15-O)
Optical method:
Near-IR spectroscopy → can detect hemoglobin saturation in DEEP TISSUE (absorbance bands for near-IR light); it's an absorption spectrophotometry method that can be used in vivo
What is the Stern-Volmer equation and what do its components mean?
I(o)/I = 1 + K(sv)[Q]
I(o) = fluorescence intensity in absence of quencher
I = fluorescence intensity in presence of quencher
K(sv) = Stern-Volmer constant
[Q] = concentration of quencher (oxygen)
In the simplest case, a plot of I(o)/I vs. [Q] should be a straight line with a slope equal to Ksv.
Equation for the Stern-Volmer constant.
Ksv = kq*t(0)
k(q) = biomolecular quenching constant
t(0) = excited state lifetime in the absence of the quencher
What is a fluorophore utilized in a fluorescence quenching experiment for the measurement of oxygen?
pyrene butyric acid
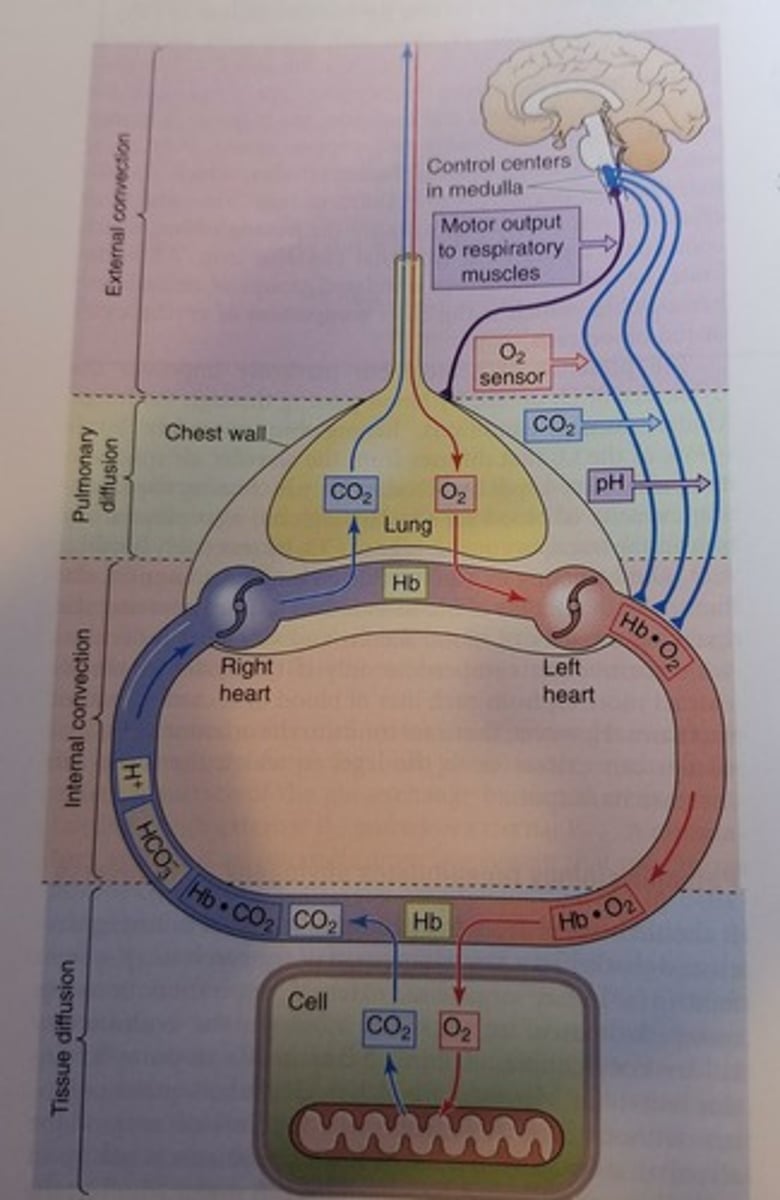
What is a Weibel diagram?
It is the general representation of a human's respiratory system.
The idea is that external convection brings in O2 in the lungs where it diffuses into the alveoli and attaches to the Hb in the blood. It travels via the left heart to the systemic tissues in the bloodstream (internal convection) where the O2 is offloaded to the mitochondria in the cells that require it. Upon metabolizing, the cells spit out CO2 which can either bind to the Hb or diffuse in the blood stream where it goes back to the right heart so that it can be pumped to the lungs, go through diffusion and ultimately expired via external convection. And then the process restarts.
What is the surface area of the lung if the alveoli were spread out?
80 - 100 square meters
What are the averages of the TLC, VC, RV, IC, and TV. And what are the general equations to identify these.
TLC = 5 L
VC = 3.5 L
RV = 1.5 L
TV = 500 mL
IC = 3.0
TLC = VC + RV
TV = VC - IC
TLC = Total lung capacity
VC = Vital capacity
RV = Residual volume
TV = Tidal volume
IC = Inspiratory capacity
What is the ideal gas law and Dalton's law of partial pressures?
Ideal gas law → pV = nRT
Dalton's law =
Total pressure is the sum of the individual pressures
What is the difference between the partial pressure of oxygen in dry and wet air at sea level?
Consider that at sea level, barometric pressure is 760 mmHg. 21% of 760 mmHg = 159 mmHg which is the pO2 at sea level in dry air.
At a water-air interface, the water vapor condenses into the air right above the water. The partial pressure of water vapor is 47 mmHg. Since Dalton's law states that the total pressure is equal to the sum of its individual parts; that means that the barometric pressure not accounting water vapor would be 760 - 47 = 713 mmHg.
21% of 713 mmHg = 149 mmHg.
So, the partial pressure of oxygen of wet air is lower than that of dry air.
What is Henry's law?
The concentration of a dissolved gas is proportional to the partial pressure of that gas.
[O2]diss = s x pO2
where is a solubility constant
What is Fick's law?
J = (C1 - C2)/X
What does the net diffusion rate depend on?
1. Distance between regions of concentrations
2. Cross-sectional area
3. Solubility of a gas
4. Molecular weight of gas
5. Temperature
Which can hold more oxygen: freshwater or salt water?
Fresh water
Which can hold more oxygen: warm water or cold water?
Cold water
At most, how much oxygen can you dissolve in 1 L of water?
7 mL
Compared to sea level, how does the barometric pressure change on Everest?
The barometric pressure on Everest is 1/3 of the pressure at sea level
What is the biggest physiological challenge for airbreathers? What do they do to combat this?
To not lose water.
O2 and CO2 are larger than water so air breathers can lose water.
They need an impermeable body coating, but with areas that allow for gas exchange.
For small animals, diffusion of oxygen works fine, but increasing size means the need for adaptation (decreasing SA:V ratio for bigger animals)
Do sponges and cnidarians need gills?
No (Cnidarians also don't have gills)
What kind of giills do salamanders have?
They have passive, external gills that have a large SA which provide the opportunity for more net flux. But they are vulnerable like this.
What kind of gills do fish and molluscs have?
They have active pump gills in which the blood and water travel in opposite directions to facilitate a countercurrent exchange.
What are the types of lungs?
1. Simple sac-like (in amphibians)
2. More complex, larger surface area, powered air pumps
a. Reptiles → septate
b. Mammals → alveolar
Fundamental difference between lungs and gills?
Lungs are invaginations
Gills are evaginations
When is the Cambrian and Precambrian eras?
Precambrian is anything before 540 mya.
Cambrian is anything 540 mya to present day
Has the oxygen percentage been stable through the last 600 million years?
No, it has fluctuated from ~10% to ~30% in the last 600 mya. There is a close correlation between extinction events and oxygen minima throughout the evolution of oxygen saturation in the atmosphere
How did oxygen potentially rise to 30%?
Hypothesis:
In the Carboniferous age (around 300 mya) oxygen is increasing, and therefore vegetation increases.
A key structural protein, lignin was being produced in trees that allowed them to grow big and tall and thrive in the high-oxygen environment. Ligninized trees were way ahead of the bacteria that were meant to degrade them, which means they would grow too tall and would often fall. These trees would get buried and not be metabolized so carbon production would be lowered while vegetation continued to increase and produce more oxygen. Of course there is a detriment that if a lightning strike were to occur, the high-oxygen atmosphere would foster an easy conflagration.
Atmospheric pressure around this time was also considerably high at around 900 Torr.
When did mammalian physiology begin to develop?
Mamallian physiology began with synapsid ancestors which were roughly 280 mya. There was a huge drop in oxygen and mammalian physiology really developed at an oxygen minima.
This is likely why mammals are so much better suited to accustoming to hypoxia rather than hyperoxia.
Briefly delineate Cambrian Oxygenation.
Cnidarians (jellyfish and anemones) → no gills, passive diffusion along entire body surface
Sponges → passive gills (but a functional pseudopump), they had a high SA/V ratio which allowed them to live in very low O2
Echinoderms (sea urchins, cucumbers) box like with outer layer of skin over skeleton, water vascular system and tube feet
Molluscs (bivalves, cephalopods)→ pump gills
Braciopods → real pump gills
Arthropods in water → segmented, jointed exoskeletons that prohibited passive diffusion which necessitated respiratory gills. Arthropods are considered the most successful phylum
Arthropods in land → "book lung". Scorpions thought to be first land animal (430 mya), millipedes and then springtails... These all died out in lower oxygen environment around 400 mya.
New wave of arthropods and animals 370 mya includes molluscs and horeshoe crabs.
285 mya O2 levels were at 30% which allowed bigger size and easier flight (think huge dragonflies and scorpions) → pO2 around 300 torr.
Terrestrial vertebrates: gills → lungs
What is Cope's rule?
Animal species tend to get larger with time as size offers protection from predation
What is carrier's constraint?
Applies to reptiles.
Breathing and running was not possible because the muscles used for running would disallow breathing
How could dinosaurs breathe and run?
They were bipedal
What are the first pre-mammals?
Synapsids, therapside, hadrocodium
How can elephants snorkel breathe and decent depths?
They don't have a pleural space that would collapse like human's would at depth
Which lung system is more efficient? A mammals or a bird's?
BIRD'S WAY MORE EFFICIENT.
33% more efficient at sea level.
200% more efficient at 15,000 ft.
Bird lungs move oxygen in one direction via n anterior and posterior sac that allows for high-oxygen air throughout the circulation; not like a bellows in the way humans do.
Also, bird lungs are 15% of their body volume vs. 7% for mammals.
Employs a countercurrent system like in fish gills.