CHM 104 Unit 1 Study Guide
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
Pure substance
matter that can be represented with one chemical formula or symbol
Mixture
a combination of two or more substances that can be separated
Homogeneous mixture
A mixture whose composition is the same throughout the sample
Heterogeneous mixture
a mixture in which the composition is not uniform throughout
Element
matter made up of only one type of atom
Compound
A pure substance made up of two or more elements chemically joined together
Period number
Horizontal row on the periodic table
Group number
Vertical column in the periodic table
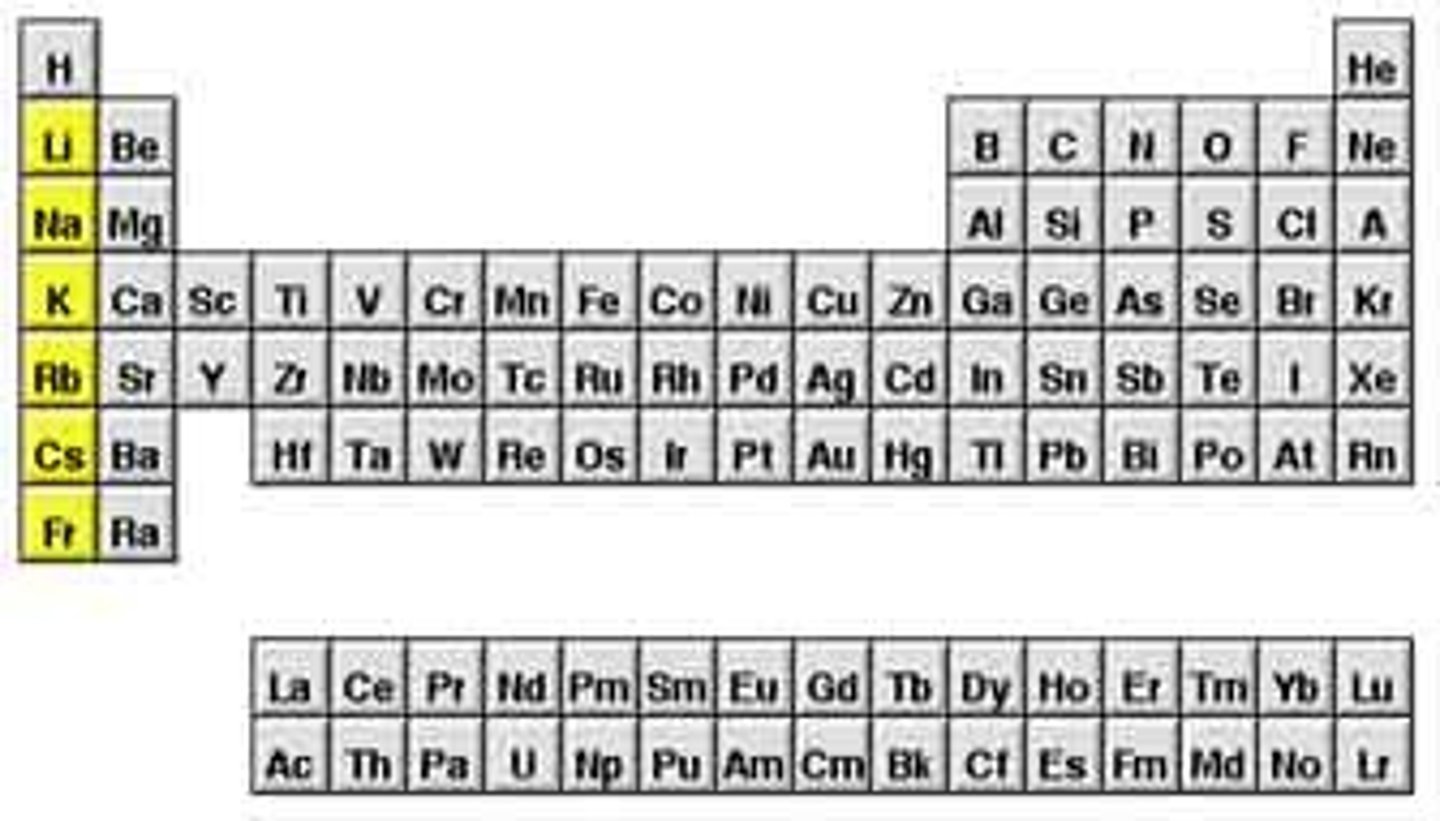
Alkali metals
Group 1A
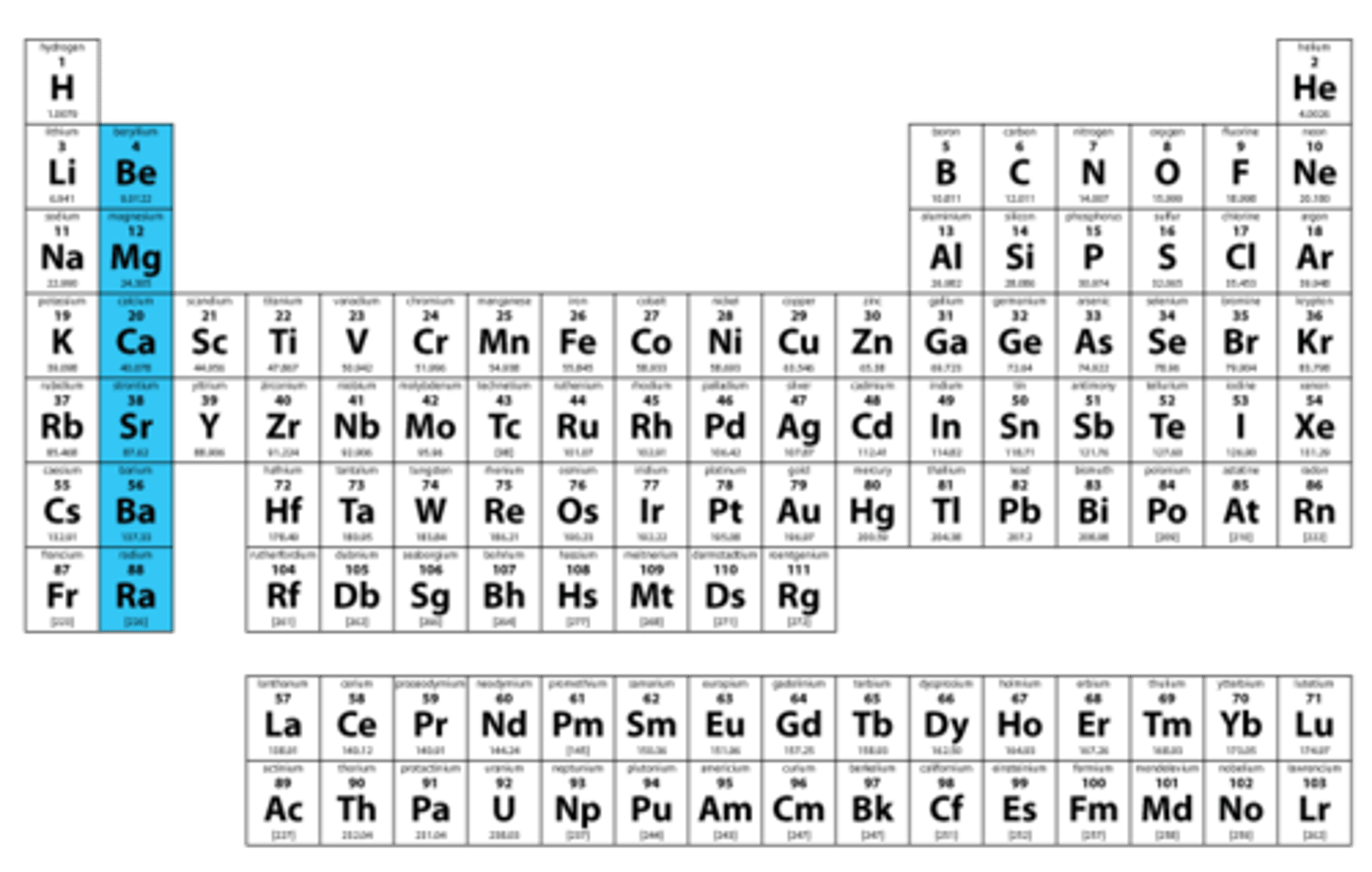
Alkaline Earth metals
Group 2A
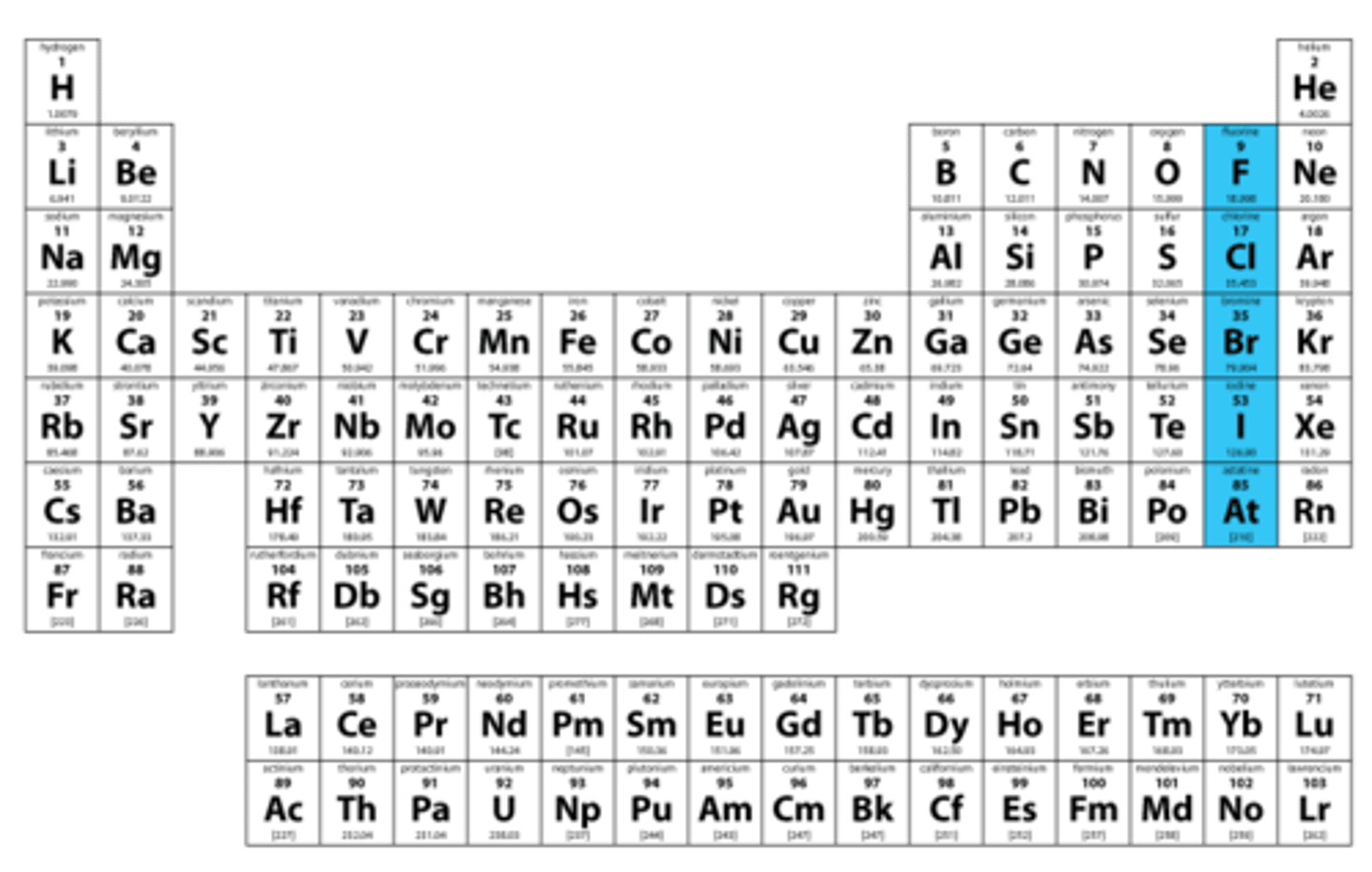
Halogens
Group 7A
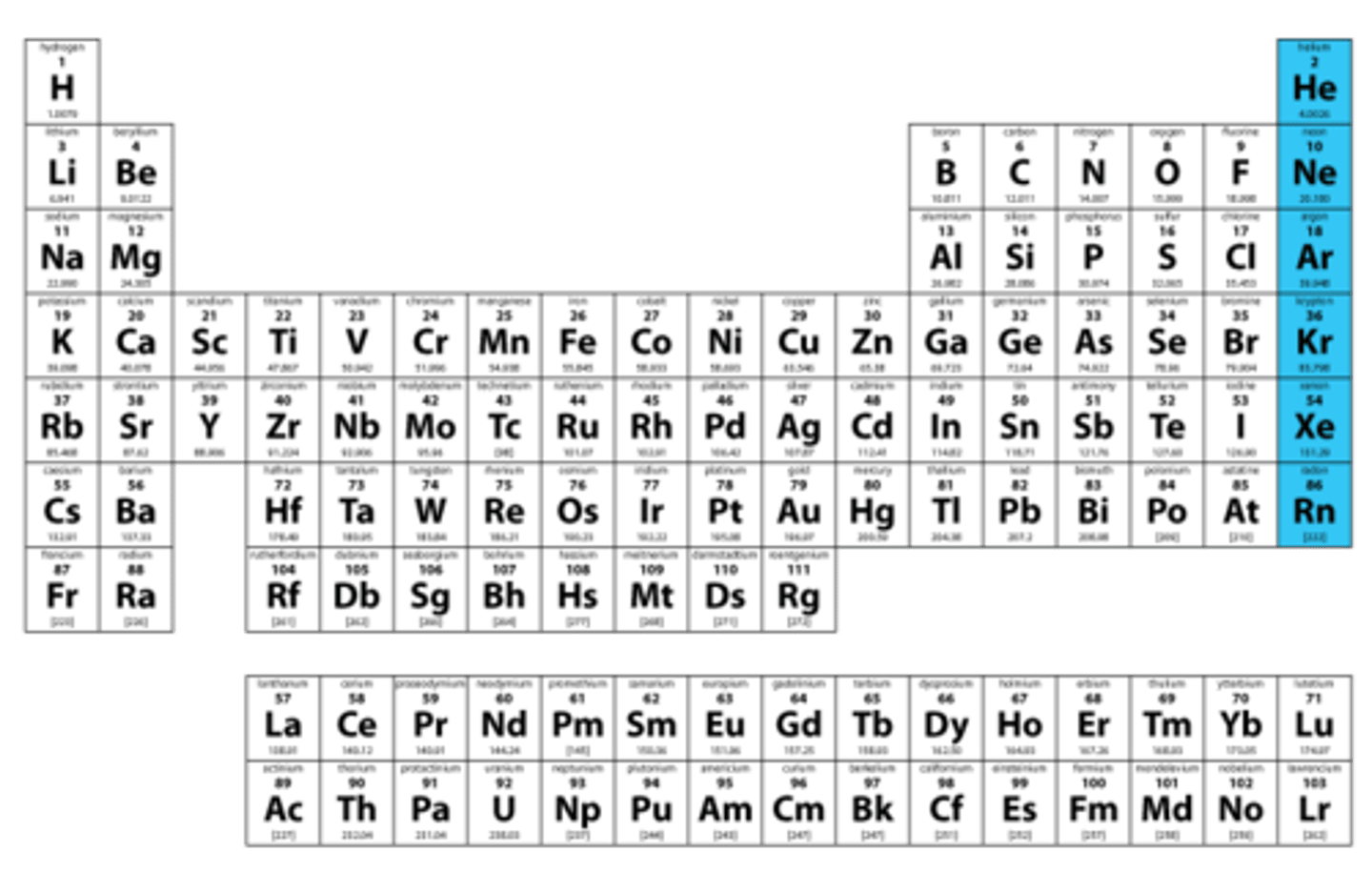
Noble gases
Group 8A
Metals
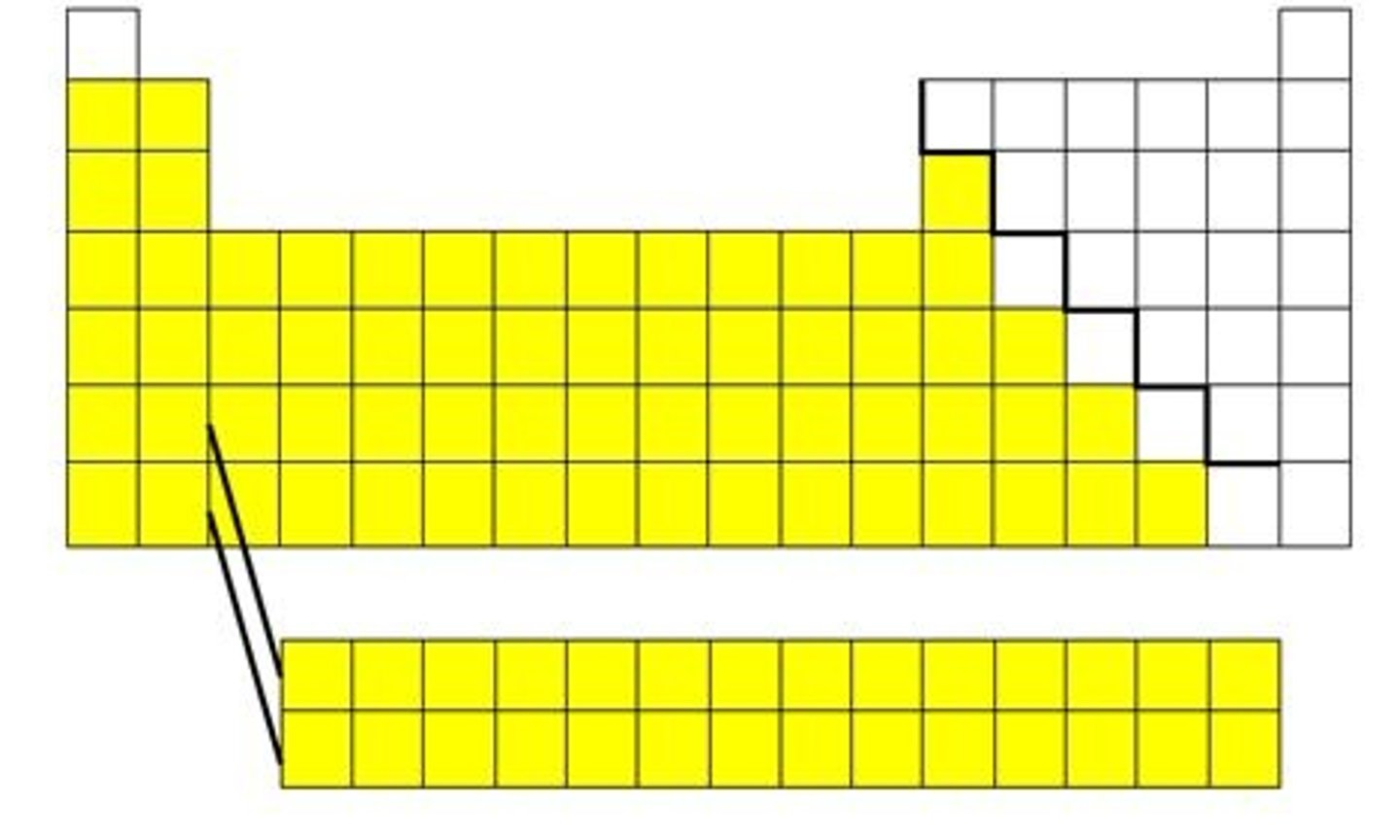
Nonmetals
Physical reaction
a change in the state of matter, while the substance maintains the same chemical identity
Chemical reaction
a change in the chemical identity of a substance
grams
unit for mass
liters
unit for volume
meters
unit for distance
grams per liter
unit for density
weight
measure of the force of gravity on an object
atom
Basic unit of matter
proton location
nucleus
proton relative mass
very heavy
proton charge
positive
neutron location
nucleus
neutron relative mass
very heavy
neutron charge
neutral
electron location
electron cloud
electron relative mass
very light
electron charge
negative