AP CHEM UNIT 2 NOTES
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
89 Terms
Electronegativity
the tendency for an atom to attract an electron while in a chemical bond (must be comparitive)
When an atom has both a high ionization energy and a high electron affinity, it will have?
high electronegativity in a chemical bond
which is the most electronegtaive ?
Flourine
the least electrongetaivte?
FrankiumFrancois
What does electronegtaivity do across a period?
increase
what does electronegativity do down a group?
decrease
mettalic
nuclei of metal atoms are attracted to delocalized electrons that move in a swarm
ionic
metals transfer electrons to a non metal to form ions that are electrotastically attracted and form a lattice structure
covalent
the oribitals in non metal atoms overlap and share electrons
non-polar covalent
the electrons are shared equally between atoms (the electrical charge is = districuted)
polar covalent
the electrons are sgared unequally between atoms (uneven districution)
partial pos charge
low electronegativity
partial neg charge
high electronegtaivuty
dipole moment
dipole arrow points toward more electronegative atom. It is a vector quantity that represents the polarity of a bond.
0-0.4 bond difference?
nonpolar covalent
0.4-1.7 bond difference?
polar covalent bond
1.7-3.3 bond difference?
ionicbond
properties of metallic
electrons delocalized from nuclei, high melting and boiling point, good conductivity, shiny, malleable
properties of ionic
electrons transferred between ions, very high melting and boiling points, conducts when melted or dossolved, brittle
polar covalent properties
electrons shared unequally, low boiling and melting points, poor conductor
nonpolar covalent
electrons shared equally, very low melting and boilin gpoint, poor conductor
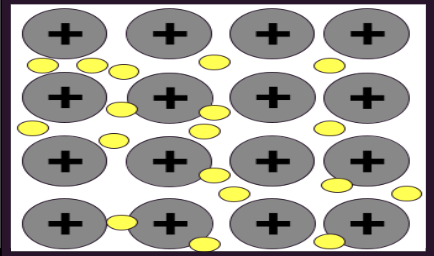
metallic

ionic
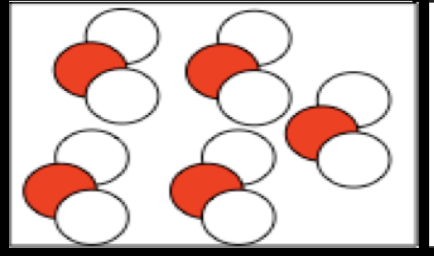
polar covalent

nonpolar covalent
why do bonds form between atoms?
decrease PE and become more stable
bond length
the distance between atoms at the point of equilibroum between repulsion and attractive forces
what happens to PE at close distances
repulsion causes high PE
what happens to pe at far distances
attractive forces increase
when is pe at minimum
the attractive and repulsive forces balance each other
bond energy
the energy needed to break a chemical bond
bonds with higher energy
have smaller bond lengths and are more stable than bonds with lower energy.
the weaker the coloumbic force and the lower the bond energy
the larger the distance between atoms
the greater difference in coulombic force and the higher the bond energy
the larger the electronegativity
bond order
the number of bonding electron pairs at a bonding sites
sigma bond
a bond forms w/ maximum overlap between orbitals (all single bonds)
pi bond
a bond forming from the overlap of two different lobes of orbitals
as bond order increases
the bond length decreases while the bond energy increases
single bond consists of
1 sigma bond - 2 electrons - 1 bond order
double bond consists of
1 sigma bond and 1 pi bond - 4 electrons - 2 bond order
triple bond
1 sigma bond and 2 pi bonds - 6 electrons - 3 bond order
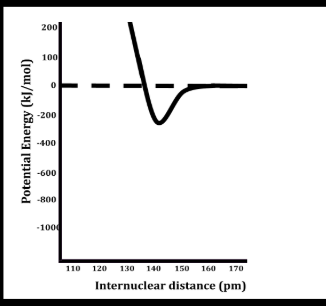
largest bond length and smallest bond energy
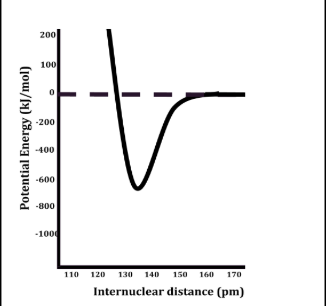
smaller bond length and larger bond energu compared to single bonds
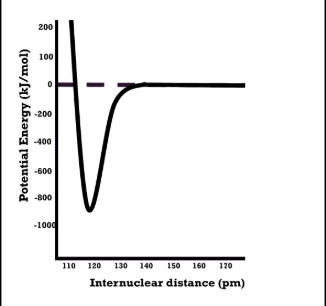
smallest bond length and largest bond energy for a bond type.
lattice energy
the energy required to separate one mole of an ionic compound into its gaseous ions. It reflects the strength of the ionic bonds within the crystal lattice.
when does lattice energy increase
the charges if ions increase, the radius of ions decreases
property of bonds with high LE
stronger/harder materials, higher melting points, lower solubility
when does the strength of a metallic bond increase
increasing cation charge and the # of valence electrons and decreasing cation radius
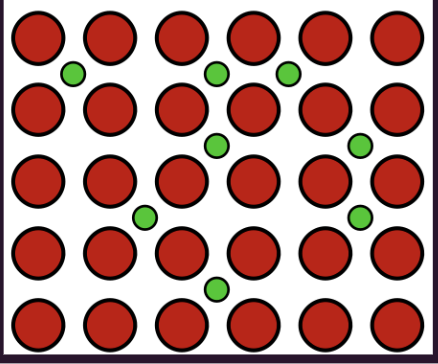
alloy
a mixture containing at least one metal
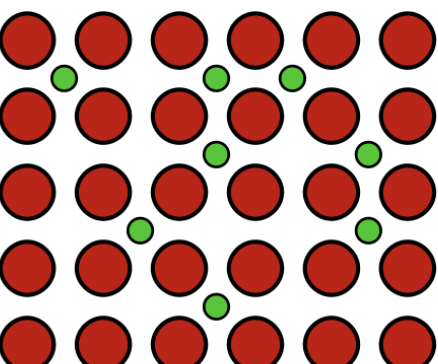
substitutional alloy
an alloy composed of atoms of similar sizes
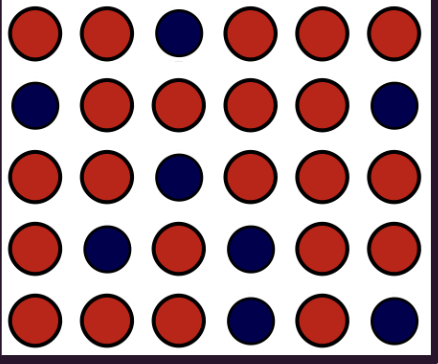
interstitial alloy
an alloy composed of atoms of different sizes
Lewis symbol
an element or ion symbol that uses a dot to represent a valence electron
odd number of electrons LDS
it is impossible to satisfy the octet rule if the number of valence electrons is uneven
incomplete octet
it isimpossible to satisfy the octet rule because the valence shell does not have a full complement of eight electrons.
a more dominant LDS structure has a?
formal charge closes to zero and the negative formal charges are on the more electronegative atoms
formal charge
the theoretical charge assigned to an atom in a molecule, based on the assumption that electrons in a bond are shared equally.
calculate formal charge
valence electrons - 1/2(bonding electrons) - nonbinding electrons
resonance
a phenomenon where a molecule can be represented by two or more valid Lewis structures, differing only in the placement of electrons.
drawing resonance
just change where double/triple bonds are
bond order
number of lines(bonds) - number of connections (to atoms)
hypervalent species
central atoms in period 3 or lower in the periodic table are large enough to bond with more than 5 valence electrons
LDS drawing tip
when there is a charge, you must subtract/add those electrons from the total to draw the striucture
VSEPR theory
the Valence Shell Electron Pair Repulsion theory, which predicts the geometry of molecules based on electron pair repulsions.
electron geometry
an area of electron densiry - depends on # of electron domains
electron domain
an area of electron density (all bonds)
a molecule is polar if
the bond dipoles unevenly cancle out
a molecule is nonpolar if
the bond dipoles evenly cancel out.
molecules w/ symmetrical geometries
are nonpolar when all the attarcted atoms are the same
molecular geometry
the three dimensional arrangement of a molecule in space
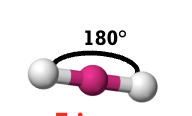
linear
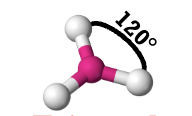
trigonal planar
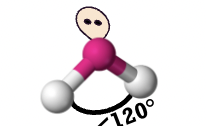
Bent
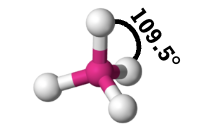
tetrahedral
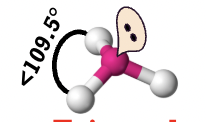
trigonal pyramidal
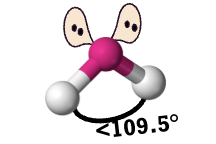
bent
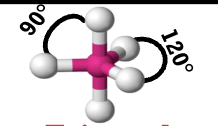
Trigonal Bipryamidal
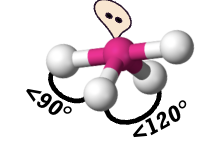
seesaw
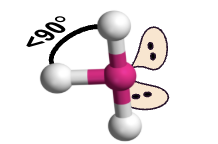
T-shaped
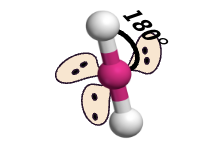
linear
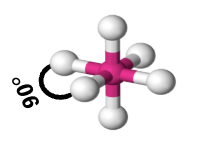
octahedral
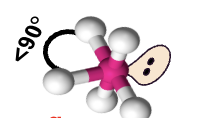
square pyramidal
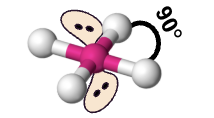
square planar
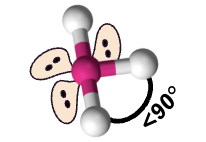
T-shaped
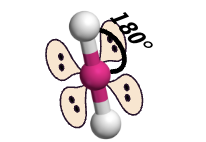
linear
hybridization
process where electron orbitals w/ similar energies mix to form new orbitals of equal energies
hybrid orbitals
oribitals of equal energy produced through the combination of orbitals on the same atom
which are stronger, pi or sigma bonds
pi bonds are weaker due to less oribital overlap, they are only present with sigman bonds
the presense of pi bonds lead to ?
structural isomers
structural isomers
compounds with the same moleculra formula but different structures