Lab Practical #1 Study Guide
1/119
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
120 Terms
Lab 1 Title
Introducing Measurements in the Laboratory
Purpose of Lab 1 “Introducing Measurements in the Laboratory”
introduce common measuring instruments to practice making measurements; learn about instrument precision
All measuring devices are subject to some error, and it is impossible to make exact measurements. (T/F)
True

What is this item called?
Metric Ruler

What is this item called?
Beaker

What is this item called?
Graduated Cylinder
Scientists record all digits of a measurement that are known exactly, plus the first one that is uncertain. These digits are collectively referred to as …
Significant digits; Significant figures; Sigfigs
Examples of Digital Instruments
Electronic Balance
Examples of Analog Instruments
Rulers and thermometers
Digital Instruments are designed to limit themselves to the correct number of significant digits, and their readings are properly recorded as given. (T/F)
True
When using analog instruments, the experimentalist is responsible for determining the correct number of significant numbers. (T/F)
True
What is the metric ruler used for in Lab 1?
to measure the dimensions of regular geometric shapes and use it to determine the area of the shapes
What were the graduated cylinder and beaker used for in Lab 1?
to measure the volume of a sample of water and compare the precision between the two
What were the triple-beam balance and the analytical (electronic) balance used for in Lab 1?
to measure the mass of an item and compare the precision between the two; to determine the mass of a powder by weighing by difference
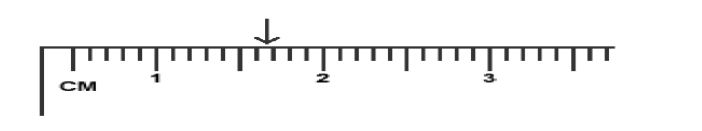
The correct reading is 1.67 cm. The first digit “1” is known exactly. The last two digits “.67” are uncertain. (T/F)
False. Reasoning: The first two digits “1.6” are known exactly while the third digit “7” is uncertain. The ruler markers are every 0.1 cm.
Measuring Liquid: When measuring liquid volumes, the graduated scale must be read from the LOWEST point of the curved surface of the liquid. (T/F)
True
What is the lowest point of the curved surface of liquid referred to?
Liquid Meniscus
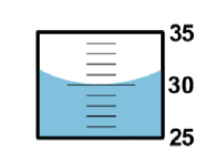
The graduated cylinder markings are every 1 mL. The correct reading is 30.0 mL. The first two digits “30” are known exactly. The last digit “.0” is uncertain. Is the last digit significant?
Yes. Reasoning: Scientists record all the digits of a measurement that are known exactly, plus the FIRST digit that is uncertain.
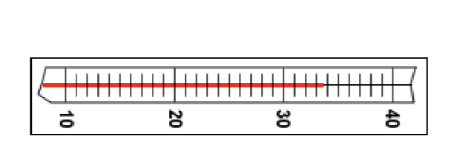
The thermometer markings are every 1 degree. The correct reading is 33.6 degrees Celsius. The first two digits “33” are known exactly. The last digit “.6” is uncertain. (T/F)
True

What is this item called?
Triple-beam balance

What is this item called?
Analytical (electronic) balance

What is this item called?
Erlenmeyer flasks; flasks

What is this item called?
Test tubes

What is this item called?
Scoopula

Accuracy
a measure of how close an experimental measurement is to the true, accepted value
Precision
refers to the degree of uncertainty in a measurement. Whichever mean of measurement that has less uncertainty, is the more precise measurement.
In general, the MORE decimal places provided by a device, the more precise the measurement will be. (T/F)
True
If multiplying or dividing measured values, the result should be reported with the lowest number of significant figures used in the calculation. (T/F)
True
If adding or subtracting measured values, the result should be reported with the lowest number of decimal places used in the calculation. (T/F)
True
How many sigfigs are in 12.548?
All 5
How many sigfigs are in 0.00335?
3 because the first zeros are leading zeros which are not significant.
How many sigfigs are in 504.70?
The last zero is a trailing zero which is significant.
How many sigfigs are in 4000?
no debate.
A track athlete ran 50.134 meters in 7.18 seconds. Her average speed is 6.98 m/sec. Why?
Because the seconds are to 3 sigfigs.
Scientific Notation N x 10^a
a standard way of writing very large and very small numbers so that they’re easier to both compare and use in computations
Scientific Notation: N is a number between 1 and 10 (T/F)
True
Scientific Notation: ^a is the number of places the decimal point was moved (T/F)
True
Scientific Notation: the exponent is negative if if the decimal is moved to the right; and if the decimal is moved to the left, the exponent is positive (T/F)
True
What’s the scientific notation of 21.72?
2.172 × 10^1
What’s the scientific notation of 0.0000475?
4.75 × 10^-5
Convert 6.09687 × 10³
6096.87
What’s the formula for area?
A=(pi)r²
Lab #2 Title
Chemistry for Biology
Purpose of Lab #2 “Chemistry for Biology”
to explore chemical reactions that take place inside an organism that are dependent upon both internal and external chemical and physical properties
Individual water molecules are constantly gaining, losing, and swapping hydrogen atoms. This process is represented by what chemical reaction?
H2O → OH + H
Water is considered to be a neutral substance. The pH of any solution can be determined by calculating the total concentration of hydrogen ions in the solution. (T/F)
True
pH scale ranges from 0-12 and the numbers represent the concentration of hydrogen ions minus 1. (T/F)
False. Reasoning: pH scale ranges from 0-14
pH scale: The more acidic the solution, the more hydrogen ions it contains (T/F)
True
Excessive changes in pH can cause metabolic and ecological problems. (T/F)
True

What is this item called?
Plastic tray with wells
Buffers
molecules that resist changes in pH and take up and release excess hydrogen ions in a solution and therefore prevent drastic changes in pH, regardless of whether acid or base is added to the solution
Where are buffers commonly found?
in dissolved minerals, soils, and in living organisms
Lab #2: What is NaHCO3?
baking soda
Lab #2: What is HCI?
hydrochloric acid
Buffers in the Blood: Bicarbonate ions act as a powerful buffer in the blood and are created when carbon dioxide reacts with water (T/F)
True
Explain how the blood is kept from reaching low pH levels.
Hydrogen ions are absorbed by hemoglobin molecules on red blood cells.
Bicarbonate ions circulate in the blood plasma, preventing rapid pH changes.
As your blood circulates past the metabolizing cells, more and more CO2 enters your bloodstream which turns into bicarbonate ions
By the time the blood reaches the lungs, it is full of bicarbonate and hydrogen ions that combine
The reaction goes from right to left, releasing CO2, which is breathed out
If a person hyperventilates, too much CO2 is removed from the bloodstream. What effect does this have on the blood pH?
The blood pH could potentially increase to dangerous levels which would result in alkalosis.
If a person holds their breath, CO2 builds up in the bloodstream. What effect does this have on blood pH?
Holding your breath makes your blood more acidic; The blood pH could decrease to dangerous levels and lead to acidosis.
Nonpolar vs. Polar Compounds: Substances that are hydrophilic (love water) are usually polar or ionic molecules themselves, and will dissolve readily in water. (T/F)
True
Nonpolar vs. Polar Compounds: Substances that are hydrophobic (hate water) are usually nonpolar molecules, and will not dissolve in water. Nonpolar substances will dissolve in a nonpolar solvent such as oil. (T/F)
True
Surfactants
special molecules that are both hydrophilic and hydrophobic. They allow water and oil to mix.
Examples of surfactants
soaps and detergents
Lab #3 Title
Basics of Microscope Skills and Cell Observation
Purpose of Lab #3
observe how the orientation of an object changes when viewed under a microscope
observe how the field of view and brightness changes when switching between low (100X) and high (400X) power
determine the diameter of the field of view under both high and low power
observe how the microscope can be used to determine the depth
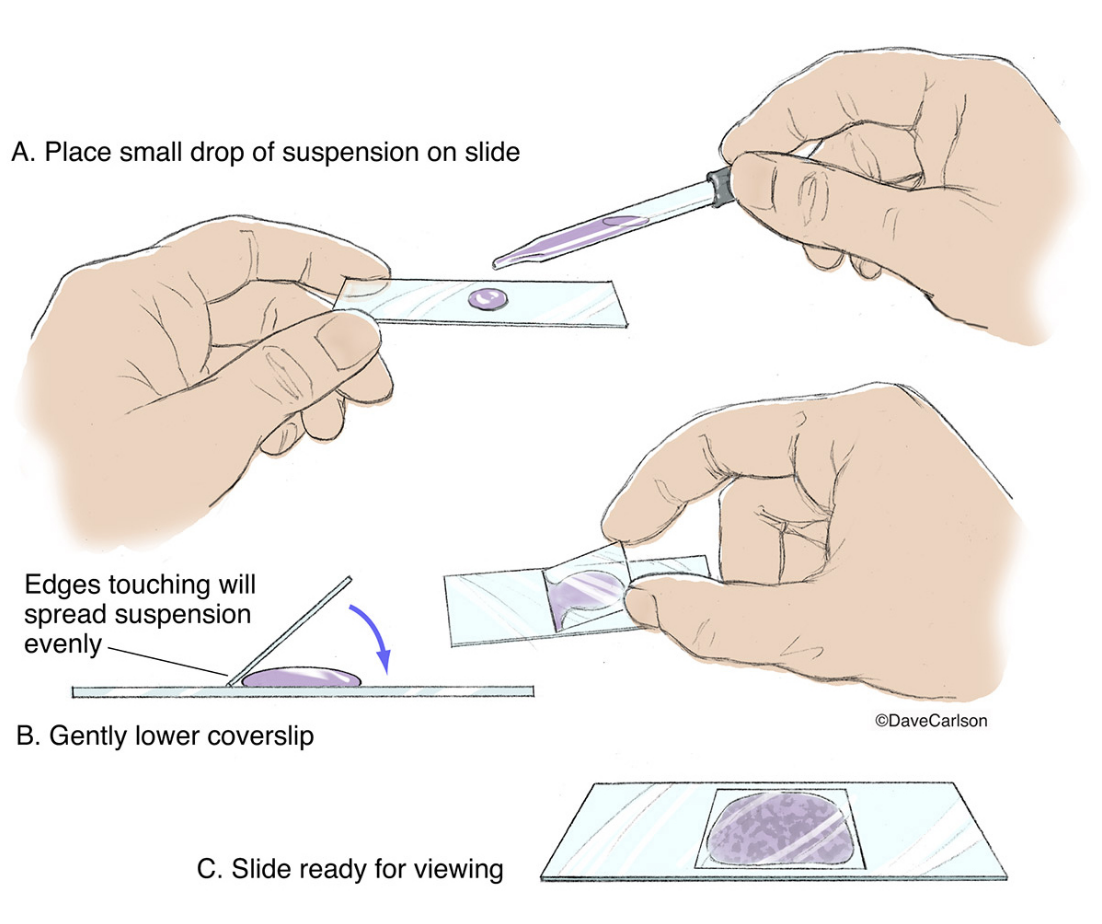
Wet mount (microscopy)
putting a drop of liquid between a slide and a cover slip
Working distance
the distance between the cover slip and the objective lens
Field of view
the circular area of light visible when looking through the microscope
Ocular Lens Magnification
10X
Objective Lens Magnification
Low Power: 4X = 40X
Medium Power 10X = 100X
High Power 40X = 400X
Oil emersion 100X = 1000X
How to find the size of the field of view
Use a clear mm ruler and observe under low power (10X objective lens)
Convert millimeters to microns
multiply by 1000
Microns are commonly used to measure cells due to their size (T/F)
True
Resolving power
the ability of the microscope to provide a clear focus, or fine detail
Depth of field
the vertical amount of the material being observed that is in focus at one time
Microscope rules
if it looks like dirt, it is
if you move the slide and what you are looking at doesn’t move, you are not looking at the slide
if it looks like a perfect circle - a dark ring with clear in the middle, it is an air bubble
if you cannot find the object, focus on the edge of the coverslip and look again
when switching objective lens, do NOT move the stage. when moving from low to high power, be careful
How would know if a cell was an animal cell?
They don’t have cell walls
How would you know if a cell was a plant cell?
They have cell walls
Why was methylene blue added to the slide with cheek cells?
It was added in order to see the organelles more clearly.
Why doesn’t the onion cell have green chloroplast?
It did not receive any light.
Would you expect to find chloroplasts present in animal cells?
No because animals don’t make their own food.
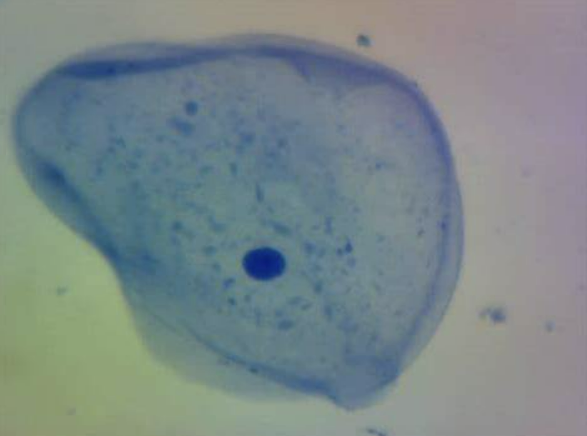
What cell is this?
Cheek cell in high power
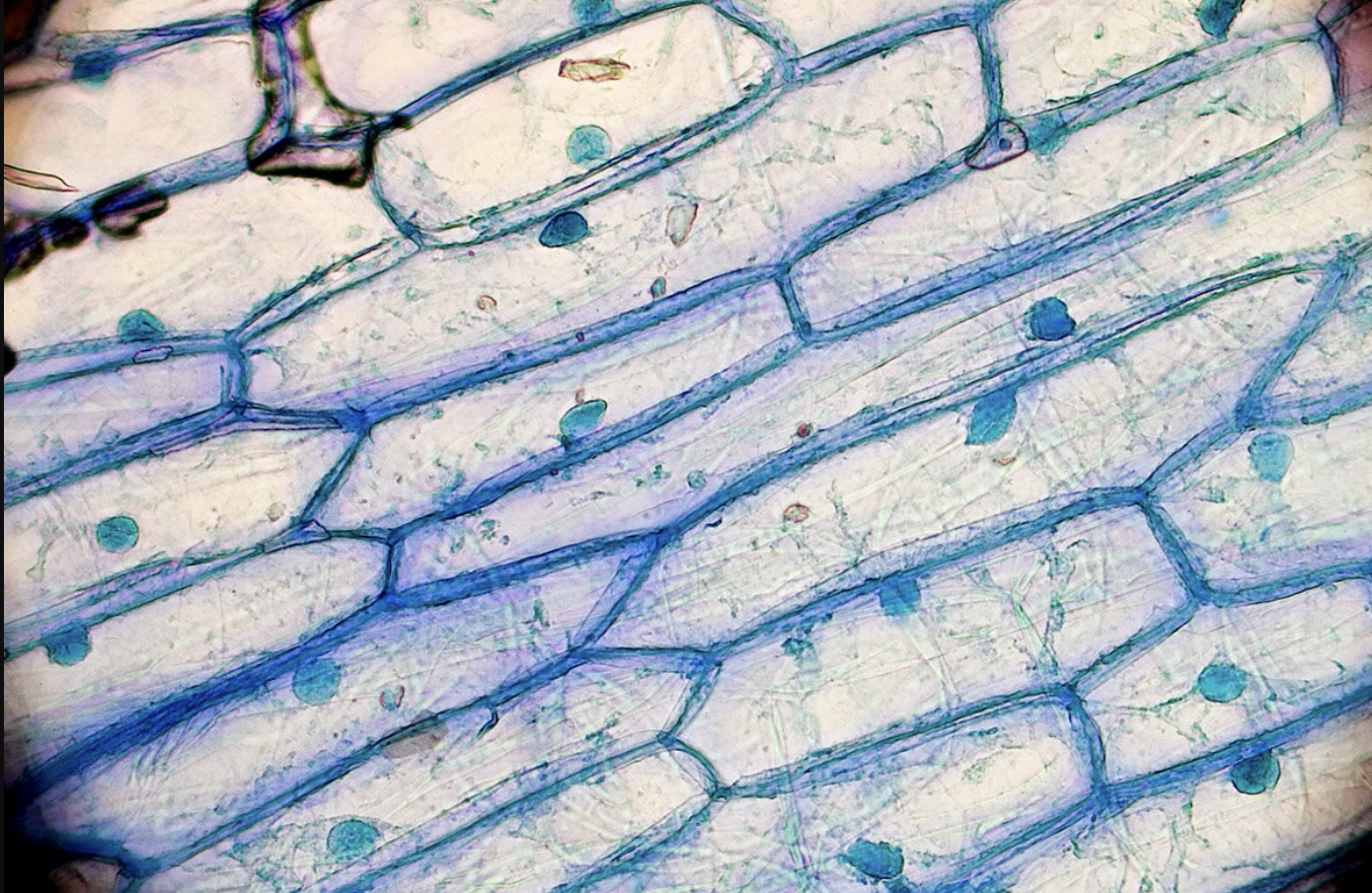
What cell is this?
Onion cell in low power
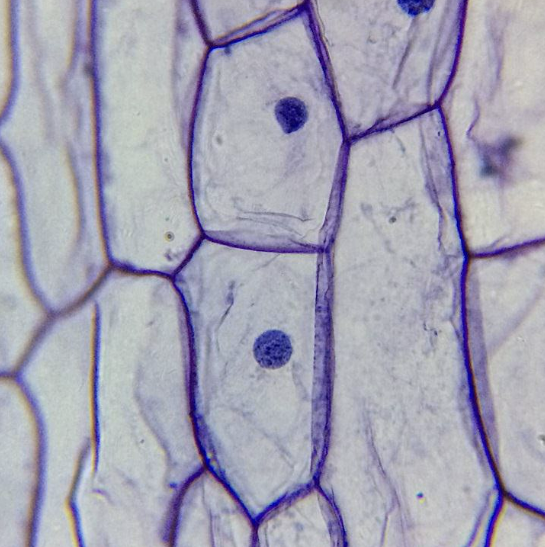
What cell is this?
Onion cell in high power
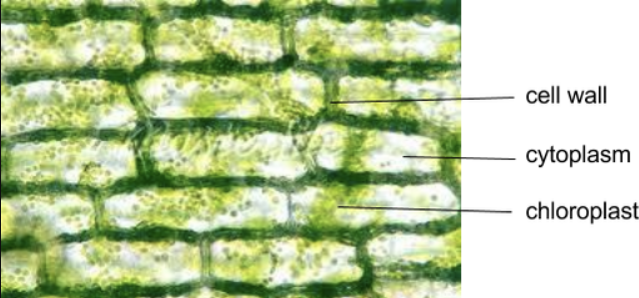
What cell is this?
Elodea leaf in high power
Lab #4 Title
You are what you EAT: How food becomes a part of you
Lab #4 Purpose
Connect the idea about the digestion of food to enzymes, polymers, and monomers
Digestive System Organs: Mouth
allows food and air to enter the body; where digestion starts
Digestive System Organs: Esophagus
transfers food from the mouth to the stomach through peristaltic movements
Digestive System Organs: Stomach
food storage organ; liquifies the food, and begins the chemical digestion of protein and fat
Digestive System Organs: Small Intestine
nearly all chemical digestion and nutrient absorption occurs here; responsible for absorbing food monomers into the bloodstream
Digestive System Organs: Large Intestine
absorbs water and salts to reduce the indigestible residue they receive, then eliminates the feces by defecation
Digestive System Organs: Liver
secretes bile which contributes to digestion
Digestive System Organs: Pancreas
produces pancreatic juices called enzymes and hormones
Carbohydrates are digested by the enzyme…
amylase which is produced by the salivary gland and pancreas
Where does the digestion of carbohydrates happen?
in the mouth and small intestine
Proteins are digested by the enzymes…
Pepsin, which is produced by the stomach and Trypsin, which is produced by the pancreas
Where are proteins digested?
in the stomach and small intestine
Lipids are digested by the enzyme…
lipase, which is mostly produced by the pancreas
Where are lipids digested?
in the small intestine
Digested carbohydrates are broken down into…
monosaccharides which convert to glucose and used to produce a fuel molecule known as ATP