Bromination Lab (Midterm)
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
Typical alkenes are __-__.
Electron-rich
Typical alkenes react with __.
Electrophiles
Addition reaction:
Bimolecular. Rate dependent on both alkyl halide and base concentrations
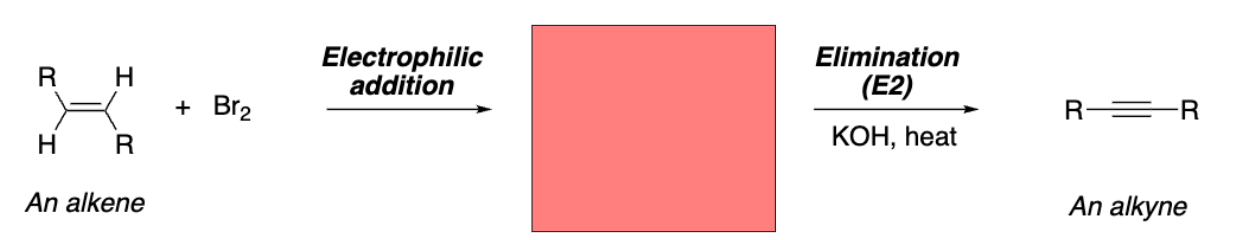
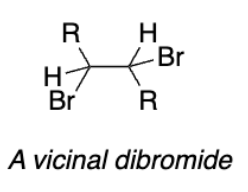
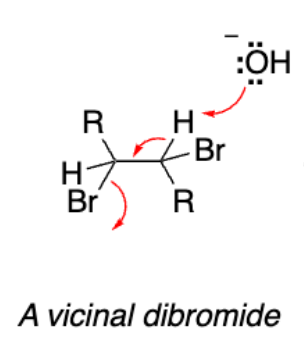
Vicinal dibromide
Elimination (E2)
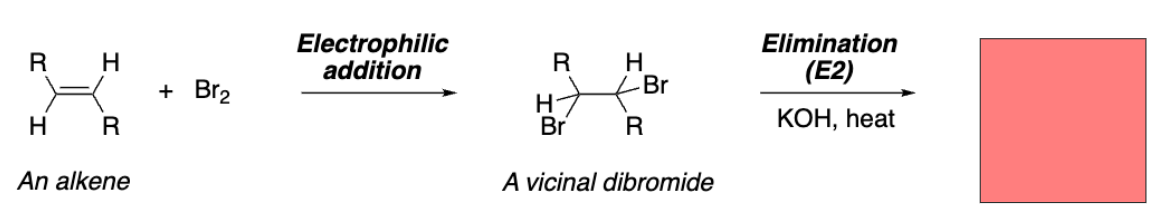
Alkyne
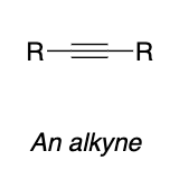
Electrophilic addition without electron withdrawing groups
H-Br as electrophile
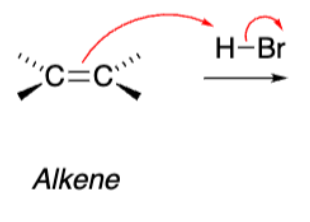
In addition of H-Br to alkene, the stereochem __ if it is __.
Inverts, Chiral
Addition reaction:
Atoms of both reactants are combined in the product
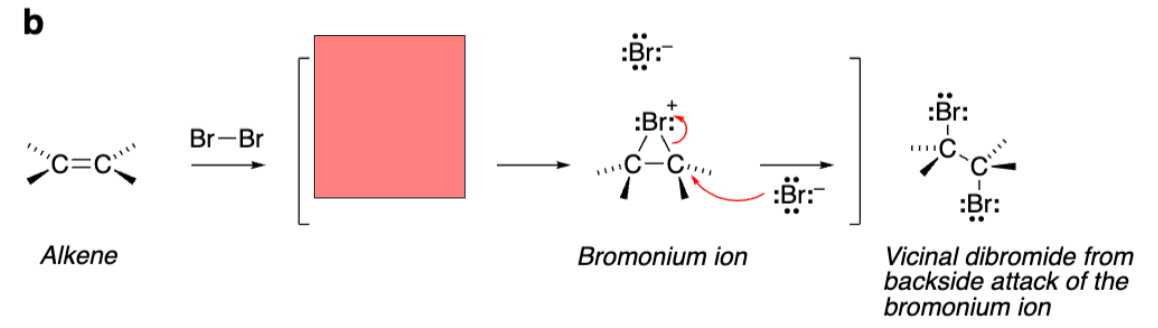
Alkene grabs one Bromine as bromine grabs alkene
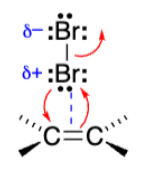
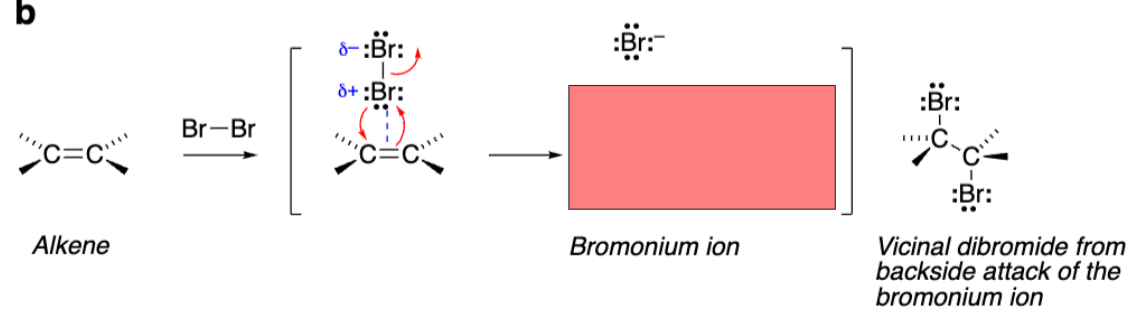
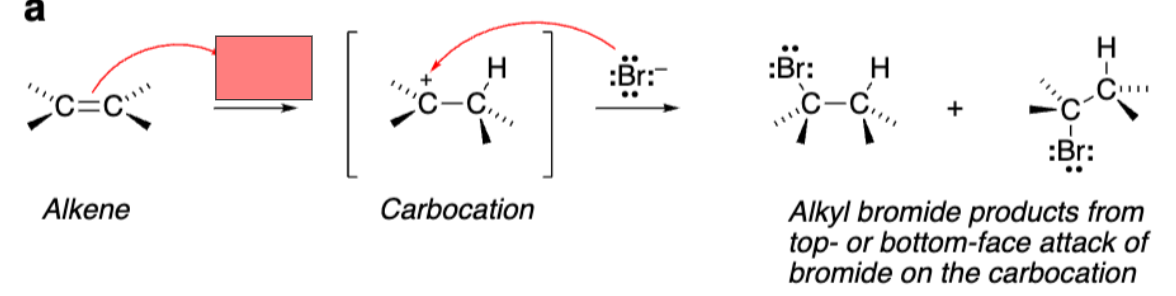
Triangle with +Bromine attached to both alkene carbons (Bromonium ion)
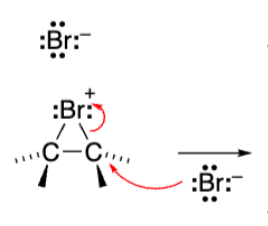
The reaction with Br2 creates a __ ion with a __ charge and not a carbocation.
Bromonium. Positive charge
Bromonium ion is ___ and reacts with nucleophiles in ___ ___ to open the ring.
Stereospecific. Stereospecific SN2 (backside attack)
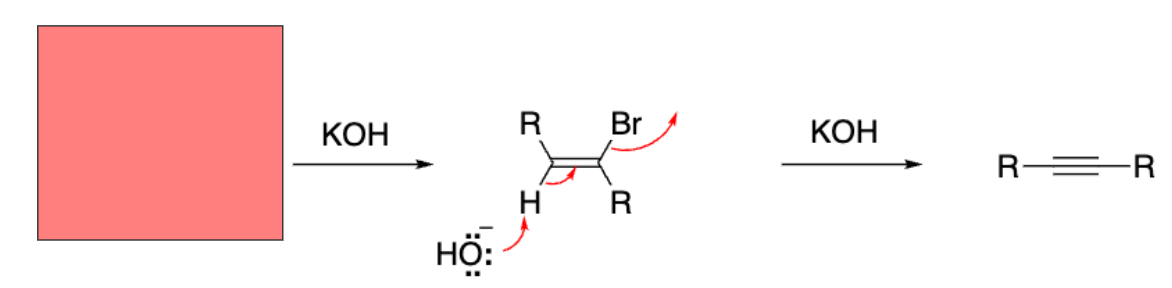
Double dehydrohalogenation
Vicinal dibromide from addition reaction
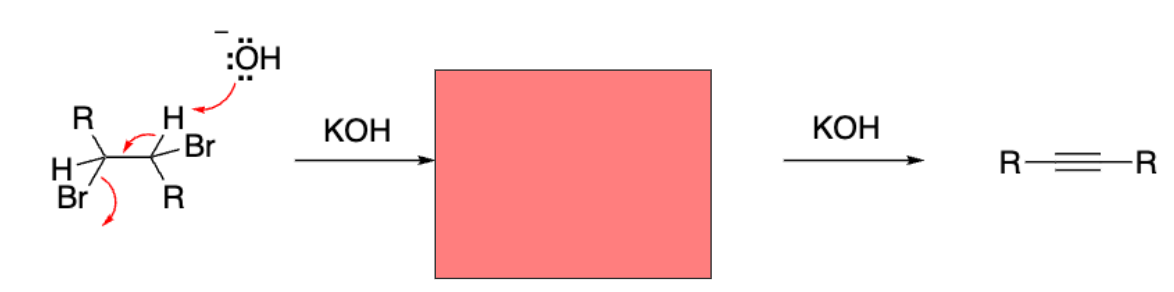
Double dehydrohalogenation
An alkenyl halide (alkene with a halide)
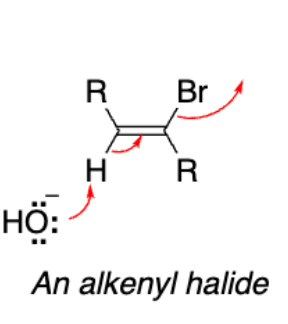
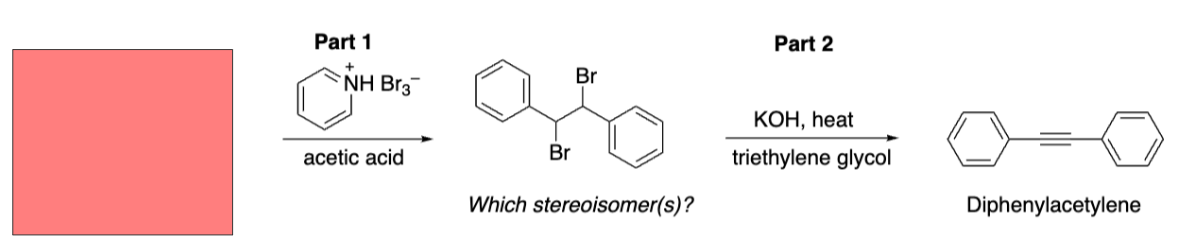
Starting material for our addition
Trans-stilbene
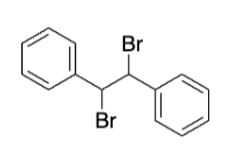
Intermediate alkane from addition before elimination
Dibromide (meso-stilbene dibromide)
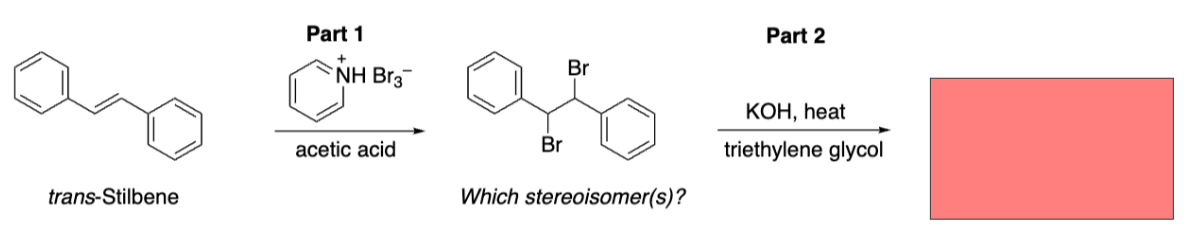
Double elimination product
Diphenylacetylene (symmetrical alkyne)
In bromination and dehydrohalogenation we used solid compound __ __ __ (__), as source of bromine, which avoids hazards of Br2.
Pyridinium hydrobromide perbromide (PyHBr3)
The elemental form of Br2 wasn’t used because it is a __ __ __.
Volatile liquid oxidant
PyHBr3 releases one molar equivalent of __ so that __ __ reactions can occur.
Br2. Electrophilic addition
Green chemistry principles used:
Low toxicity - solid form of Br2 to minimize vapors of Br2
We performed a __-induced double __ reaction of a vicinal dihalide.
Base-induced double elimination
Carbons of alkenes…
Sp2 hybridized with leftover p-orbital with one electron
Leftover p-orbitals of two carbons can combine to make…
New molecular orbitals / HOMO and LUMO
The reaction would occur more readily with the racemic chiral dibromide since the leaving group (Br) and hydrogen are ___. For an E2 elimination ___ is more favorable which makes occur readily.
Antiperiplanar (x2)
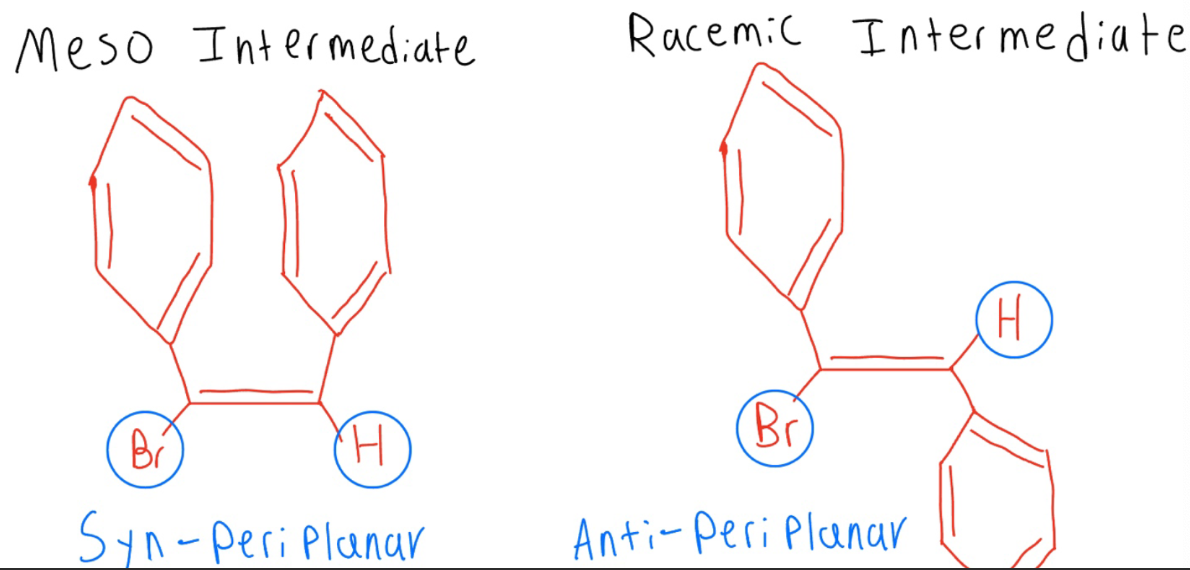
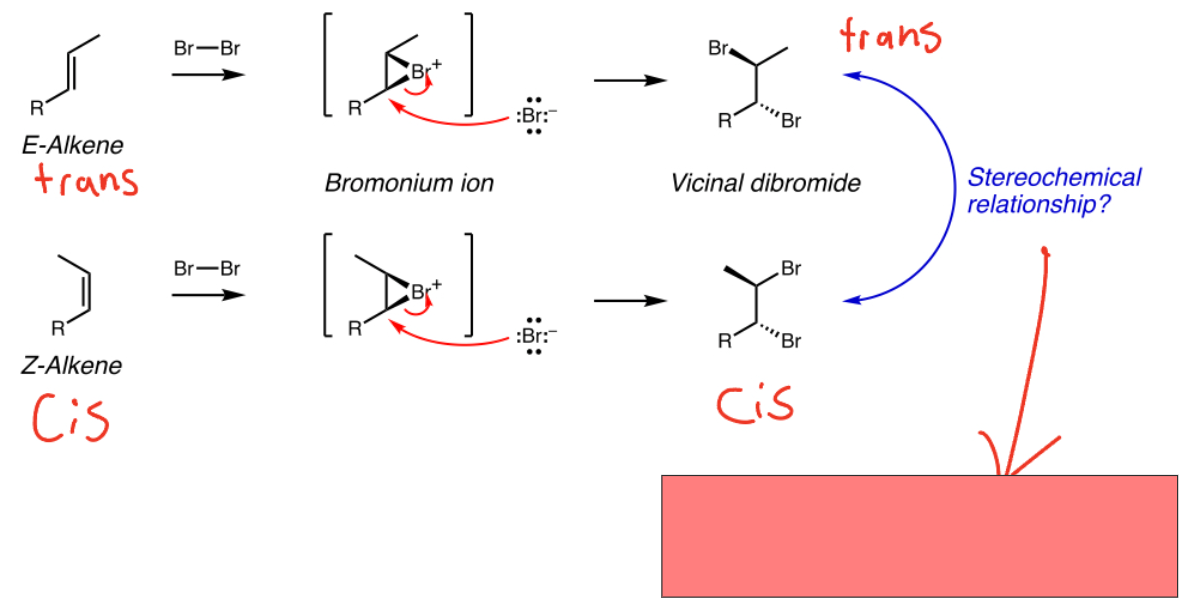
Bromonium ion product relationship
Diastereomers. Stereochem around alkene Carbons is retained/stereospecific (cis-cis, trans-trans)
Triple bond of an alkyne appears in IR spectrum for ___ alkynes.
Unsymmetrical
The triple bond frequency in IR is _____.
2100-2260 cm-1
Strong __ initiate dehydrohalogenation. Concerted reactions __ depend on __ __.
Bases. E2 bimolecular reactions depend on antiperiplanar hydrogens
The diphenylacetylene’s alkyne stretch is __ expected to show up on the IR spectrum.
Not expected