Lecture 4: (Nomenclature) Naming Compounds & Rules Chapter 3 [1.29.26]
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Nomenclature
the systematic set of rules for naming chemical compounds, ensuring every substance has a unique, descriptive name that reveals its structure
Compounds can be divided into two categories
Ionic and Molecular
Cations
(positive ions) Metals, they lose electrons. (Everybody wants to be like the nearest noble gas. EX: Magnesium forms a 2+ cation. Why? Magnesium is identified by its # of protons, which is 12. The nearest Noble gas to Magnesium is Neon, #10. 12-10= +2 charge).
Anion
(negative ions) Nonmetals, they gain electrons.
Ionic Compound
Metal + Nonmetal, EX: NaCl.
(ionic compounds transfer valence electrons from one atom to another, resulting in the formation of charged ions that attract each other).
Molecular Compound
Nonmetal + Nonmetal, EX: H2O. These are combined by covalent bonds (sharing valence electrons). This sharing allows nonmetal atoms to achieve a stable outer electron shell, creating distinct, neutral molecules.
Valence Electrons
are the specific electrons in an atom's outermost shell, making them key for chemical bonding. Core electrons are tightly bound to the nucleus, but valence electrons are loosely held and easily gained, lost, or shared to form bonds, dictating an element's chemical properties. (All valence electrons are electrons, but not all electrons are valence electrons).
Ionic Compounds can be split into two categories
Binary Compounds and Polyatomic Compounds.
Binary Compounds
Only 2 Types of Elements. The Rules for Naming Ionic Binary Compounds: Name of Metal + Base Name of Nonmetal + ide. EX: NaCl = Sodium chloride
Rule for Naming Ionic Binary Compounds
Name of Metal + Base Name of Nonmetal + ide. EX: NaCl = Sodium chloride
Polyatomic Ion
groups of two or more atoms covalently bonded together that act as a single unit and carry a net electrical charge (positive or negative). EX: NH4+ (ammonium).
Polyatomic Compound
substances composed of a metal cation and a polyatomic anion. The Rules for naming a Polyatomic Compound: Name of Metal + Polyatomic ion. EX: NaPO4 = Sodium phosphate
The Rules for naming a Polyatomic Compound
Name of Metal + Polyatomic Ion. EX: NaPO4 = Sodium phosphate
Polyatomic Oxyanions.
a negatively charged group of two or more atoms, including at least one oxygen atom, held together by covalent bonds, that acts as a single unit. EX: NO3- = Nitrate. (BASICALLY A POLYATOMIC ION THAT CONTAINS AN OXYGEN ATOMS. THE RULES FOR NAMING THESE SPECIFIC POLYATOMIC OXYANIONS ARE DIFFERENT THEN POLYATOMIC IONS THAT DO NOT CONTAIN OXYGEN).
Rules for Naming Polyatomic Oxyanions
Name of Metal + Polyatomic Ion. MORE = ATE, MORE THAN MORE = PER (more than -ate) LESS = ITE (less than the -ate form), LESS THAN LESS = HYPO (less than the -ite form). (THIS ONLY APPLIES WHEN NAMING POLYATOMIC OXYANIONS BECAUSE THEY VARY IN OXYGEN ATOMS).
Variable Charge Metals (most are transition metals)
elements that can form ions with different positive charges (they don’t have a fixed charge like most elements do). Rules for naming Variable Charge Metals: metal name + its charge in Roman numerals within parentheses + the anion's name (nonmetal) with an "-ide" suffix. EX: Au+3 + S2- = gold(III)sulfide
Rules for Naming Molecular Compounds
Prefix and Name of 1st element + Prefix and Base Name of 2nd element + ide. (When the second element contains only 1 atom, you can write mono as its prefix. However, do not write mono as a prefix for the first element, even if it only contains one atom). EX: NO2 nitrogen dioxide. H2O dihydrogen monoxide.
Prefixes for the first and second element (based on the subscript) are only used when naming
Molecular Binary Compounds
When writing the correct chemical formula…
(use criss-cross method) or determine what the others charge would be for the compound to be neutral. EX: Mg2+N3- = Mg3N2 this could have been solved by using the criss cross method (easiest and quickest way).
A compounds overall electrical charge is…
neutral. This is why it is important to write compounds so that the charge equals 0. EX: Magnesium (Mg forms a 2+ charge) and Nitrogen (N forms a 3- charge) in its raw form is Mg2+ N 3- however, because they both contain charges (positive and negative) we must make the overall charge 0. To do this, we rewrite it as Mg3N2 (solved using criss-cross method).
Acid
An acid is a substance that produces hydrogen ions in water, has a sour taste, and a pH less than 7.
Acid Solutions can be divided into two categories
Binary (begins with hydro) and Tertiary (contains hydrogen but the prefix hydro is not written as a prefix)
Most Acids start with…
H (hydrogen) however H is referred to as a proton. Because it only has 1 proton. So H = proton
Binary Acids
Binary acids are simple acids composed of only two elements: hydrogen and one non-metal element (usually a halogen like Chlorine, Fluorine, Bromine, Iodine, or Sulfur).
Rules for Naming Binary Acidic Solutions (H and X)
Hydro + base name of X + ic + acid. EX: Hcl: Hydrochloric acid. (when it comes to naming acids, the subscript doesn’t affect it. EX: H2S is hydrosulfic acid.
Tertiary Acids
H + Polyatomic Ion. EX: HNO2 = Nitrous Acid (Do not write hydro as a prefix.)
Rules for naming Tertiary Acids
H + Polyatomic Ion. Do not include the prefix hydro. If the polyatomic ion ends in ate, replace it with ic. If the polyatomic ends in ite, replace it with ous. EX: HPO4 phosphate becomes phosphoric acid.
Solution
A homogeneous mixture of two or more substances in which a solute is uniformly dissolved in a solvent.
Solute
The minor component in a solution, dissolved in the solvent. EX: American liquor contains 40% ethanol (alcohol) and 60% water. The solute is the ethanol.
Solvent
(The Major Component) a substance, typically a liquid, that dissolves another substance (the solute) to form a uniform solution. EX: Russian liquor contains 60% ethanol (alcohol) and 40% water. The solvent is the ethanol.
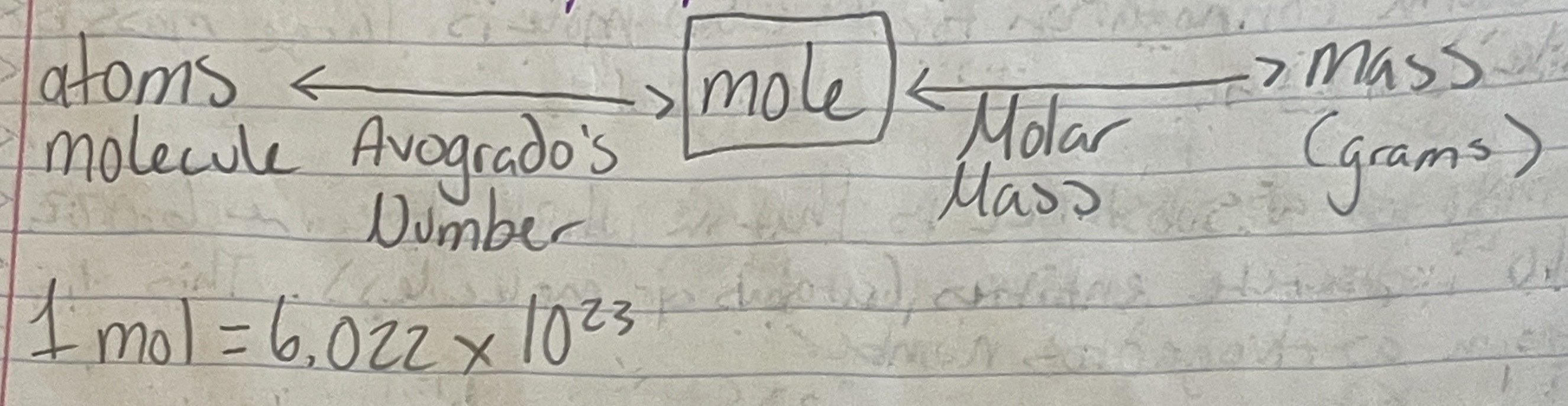
Going from Atoms to Mass (g)
When going from Atoms to Mass, use the following pathway (SEE IMAGE). If you want moles from atoms, use avogadro’s number, if you want mass (g) from moles, use molar mass (vice-versa).