biological membranes
1/77
Earn XP
Description and Tags
phospholipid intrinsic and extrinsic proteins cholesterol fluid mosaic model external and intracellular membranes beetroot practical standard deviation
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
78 Terms
structure of a phospholipid
1 hydrophilic phosphate grp (neg charge so water soluble)
1 glycerol
2 hydrophobic fatty acid tails (uncharged and non polar so water repelling )
how are phospholipids arranged in the plasma membrane ?
the hydrophilic phosphate heads face the aqueous extracellular space (tissue fluid ) and the aqueous cytoplasm while the hydrophobic fatty acid tails face inwards away frm the aqueous solutions forming a phospholipid bilayer
what happens to phospholipids when submerged in water ?
they form micelles
what is diffusion ?
the net movement of particles from an area of high conc to a low conc down a conc gradient
passive process
why is diffusion a passive process ?
it never requires energy from the cell as the particles alr have kinetic energy
which types of particles can pass through the phospholipid bilayer via simple diffuison
small non polar and lipid soluble e.g. steroids molecules
why can water diffuse directly through the bilayer despite being polar ?
its small so it doesnt come into direct contact w/ the hydrophobic tails
why cant large polar molecules diffuse directly through the bilayer
theyre rejected by the hydrophobic fatty acid tails which also form an impermeable barrier to large molecules
how do charged ions pass through the bilayer
via facilitated diffusion - passive
they must pass through channel proteins
bind to a specific bonding site down their conc gradient
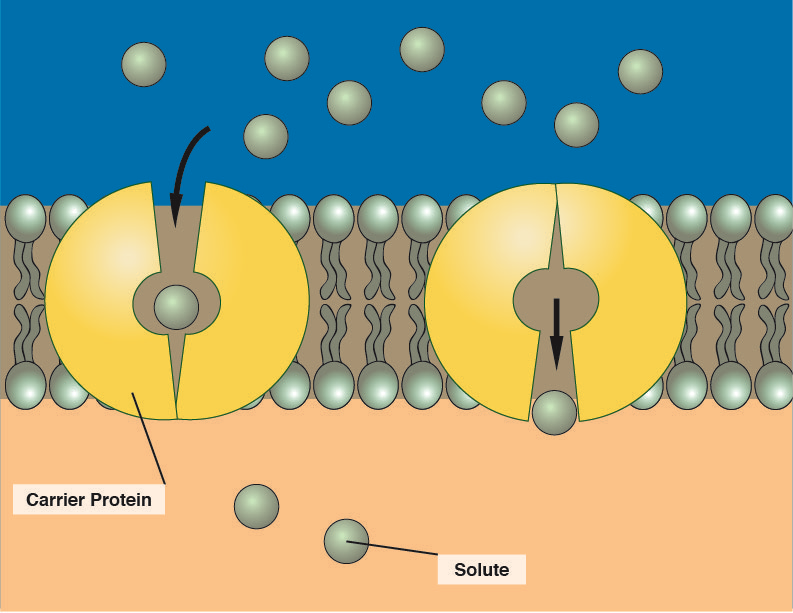
how do large polar molecules that are not ions pass through the bilayer ?
via facilitated diffusion - passive
molecule binds to binding site on carrier protein
carrier has an allosteric change in shape
molecule is released on the other side of the membrane
moves down the conc gradient
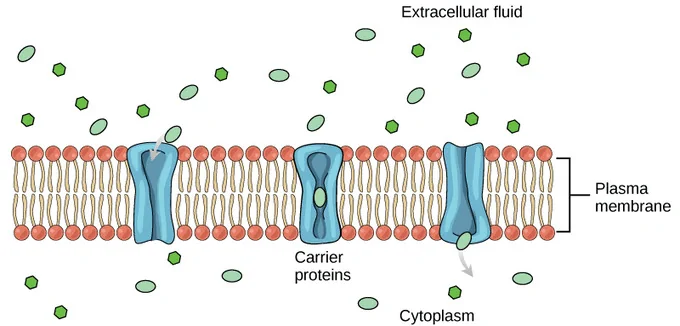
channel protein structure
intrinsic protein
spans entire bilayer
hydrophobic R grps face outwards to interact w/ hydrophobic fatty acid tails allowing for stability
hydrophilic R grps face inwards allowing molecules to diffuse through
structure of the bilayer and what it contains
7nm thick
extrinsic and intrinsic proteins
glycoproteins e.g. glycocalyx for cell recognition so cells grp tg to form tissues
extrinsic proteins structure and functions
spans one part of the bilayer
cell signalling
Receptor sites where drugs, hormones and antibodies bind triggering chem reactions within the cell
cell signalling in extrinsic proteins
cells communicate w/ each other to control processes inside the body and respond to changes in the environment
one cell releases a hormone which travels in the blood to another cell
hormone detected by another cell as it binds to specific complimentary receptor on cell membrane of extrinsic protein
creating an up cascade of chem reactions within the specific cell
glycoprotein
protein + sugar (branch of carb )
glycolipid
sugar + fatty acid tails
glycolipid and glycoproteins functions
receptor sites where drugs hormones and antibodies bind triggering chem reactions within the cell
cell signalling
act as antigens
have OH and H grps that can form H bonds w/ surrounding molecules
cholesterol structure
sits between phospholipid tails in both layers w/o it cells not supported esp if its not apart of a tissue e.g. rbc
cholesterol function
maintains stability and fluidity of the membrane as it binds to fatty acid tails packing them closer tg
at body temp - holds fatty acid tails tg to make cell less fluid
in icy areas - prevents crystallisation by inc fluidity (as the cell would otherwise shrink and become impermeable ) allowing cell to survive and function
what can inc the fluidity of the cell surface membrane at low temps ?
C=C double bonds in the fatty acid chains bc they kink the chains
fatty acids having shorter chains
external ( cell surface ) membranes
control what comes in and out
cell recognition (antigens )
cell communication ( cell signalling )
intracellular /membranes within cells
membranes around organelles
compartmentalisation (keep spec conditions needed e.g. in rER )
form vesicles to transport substances to diff areas of the cell
sites of chem reactions ( e.g. inner membrane of mito contains enzymes for respiration )
barrier between organelle and cell controlling movement of substances in and out of the organelle
describe the fluid mosaic model of the plasma membrane
fluid bcs the proteins can move freely through the bilayer phospholipids can sway side to side
mosaic pattern from above formed by the scattered proteins
model bcs its a visual created based on experimental and chemical evidence
the ease by which they do this depends on the num of phospholipids w/ unsaturated fatty acids in the phospholipids as more = more fluidity
why is facilitated diffusion passive ?
particles alr have KE and are moving down the conc gradient
how do bacteria maintain their shape if they have no cholesterol ?
murein cell wall which helps to maintain their shape
4 factors that affect the rate of diffusion ?
SA
Diffusion distance
conc gradient
temp
how does an inc in temp affect the rate of diffusion ?
incs KE of molecules and speeds up diffusion of particles
how does an inc in SA affect the rate of diffusion ?
the num of particles that can diffuse at one time incs so rate of difusion incs
how does an inc in the steepness of the conc gradient affect the rate of diffusion ?
steeper conc gradient = faster rate of diffusion
how does an inc in the diffusion distance effect the rate of diffusion ?
larger distance = slower rate of diffusion

what does Ficks law state ?
Rate of diffusion is:
proportional to the SA
proportional to the conc gradient
inversely proportional to the diffusion distance
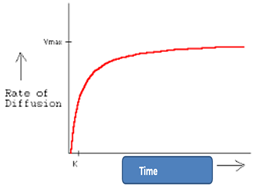
explain the graph
the conc gradient at the beginning must be steep in order for diffusion to occur
As time incs w/ more substances moving down the conc gradient the steepness is reduced so the rate of diffusion decreases till equilibrium is reached so there is no longer a conc gradient.

explain the graph
Linear graph line
As the conc gradient incs the steepness of the conc gradient incs
Diffusion incs in a linear form.
According to fick’ s law the rate of diffusion is proportional to conc gradient therefore the rate incs
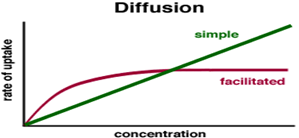
describe the graph that relates to facilitated diffusion (conc of substance)?
as the conc incs initially the rate of diffusion also incs (as conc must be a LF)
When the conc of the substance continues to inc the steepness of the conc gradient incs
The graph will plateau and the rate will reach saturation (v max) as the number of protein / carrier channels are saturated. This has become a limiting factor
active transport process ?
particles move from a low conc to a high conc against the conc gradient
active process so needs ATP and carrier proteins
facilitated diffusion vs active transport
both :
carrier proteins
involve the movement of particles using an intrinsic protein
FD :
channel proteins
no ATP - passive
down the conc gradient
AT:
no channel proteins
ATP - active
against conc gradient
in a beetroot why cant betalain diffuse out of the membrane
too large to fit in between the tails of the fatty acids
polar so rejected by fatty acid tails
beetroot practical method
use a cork borer to cut out pieces of the beetroot from the same source variety and age
use a ruler and a sharp scalpel to cut these into 5mm discs all with the same SA
place all discs into small beaker and wash under running water for 5 mins to remove excess pigment
label 7 test tubes with temps 20-80
measure 5cm³ of distilled water w/ a measuring cylinder and add into boiling tubes
make a water bath using large beaker and water frm a kettle
when the water bath reaches 30 using a thermometer place beetroot peices in boiling tube then put boiling tube into water bath
after 30 mins stir same num of times go no more than 60 mins
what are the control variables in the beetroot practical?
sa of discs
source for same conc of pigment
same variety and age for same conc/vol of pigment and steepness of conc gradient
temp - water bath at 25 as too low to denature proteins
precautions in the beetroot practical
rinsing the discs to remove excess stain bcs it produces an over estimation in absorption and shows membrane has incd fluidity / more permeability than it rlly is
shake the curvette before taking absoprtion reading - distribution of pigment precise measurement
wash piece of appratus and get new one each time - prevents contamination
how could the data be made more precise in the beetroot practical instead of judging absorbance by observing the colour ?
pour the solution into a cuvette and put into colorimeter
choose green wavelength
add the distilled water in a cuvette to make sure absorbance is 0 - this is a control
repeat process 3 times for each temp to allow anomalous data to be identified
controls / precautions when making data more precise in beetroot practical
water in colorimeter ensures all values r measured to the same standard and comparable incs validity
repeats produce a mean value or statistic test e.g. SD to inc reliability
limitations of beetroot practical and solutions
colorimeter can only detect small range of absorbance - theres a definite max absorbance that can be detected so darker solutions js give same reading
solutions:
dilute each sample before placing in cuvette
use smaller num of discs per boiling tube
what factors damage a membrane
temp
pH
ethanol
detergent
how does temp damage the membrane ?
phospholipids move round more no longer held in a tightly gelled state
membrane becomes more fluid and permeable
pigment begins to diffuse out
steepest gradient indicates higher temp so phospholipids gain even more KE and begin to melt
membrane becomes even more fluid and permeable
h bonds vibrate due to incd KE , they break , proteins denature
membrane breaks down -most permeable atp so most pigment -which has greatest KE- diffuses out w/ fastest rate of diffusion
how does pH damage the membrane
when the pH is away from the optimum:
too acidic (H+ions )
too alkali (OH- ions )
these ions break ionic and H bonds in the tertiary structure so proteins denature
cell membrane breaks down bcs of incd permeability and all pigment diffuses out
how do ethanol and detergent damage the membrane and what does the plateau on the graph showing this mean ?
ethanol -non polar and fat solvent so more ethanol = more phospholipids dissolve membrane becomes more permeable so pigment diffuses out
plateau means :
equilibriums been reached or if membranes fully dissolved atp then all pigments js leaked out
membrane more permeable and fluid
in the beetroot practical when using ethanol or pH state independent and dependent variables
independent - conc of ethanol ( 5 diff concs so 0-2mol dm^-3 ) serial dilutions to get diff concs
or
pH in which case pH buffer and probe are used
dependent - absorption of the solution surrounding the beetroot (A.U)
what is standard deviation ?
a measure of the spread around the mean
what does it mean when theres no overlap between standard deviations and theyre little?
low sd shows results r consistent w/ little variation
no overlap between sd bars means there is a likelihood of a sig diff between results so data is repeatable and precise - greater certainty
what does it mean when theres overlap between standard deviations as theyre large?
large sd shows variable results
overlap between sd bars shows theres a likelihood of no sig difference between data so less certainty as data is unrepeatable unprecise
why is sd better than range for dispersion of data
sd is less affected by anomalies than range and also takes into acc all values
water potential
measure of the ability of water to leave a solution - pressure so in kPa
water potential of pure water and why ?
highest possible water potential of 0kPa bcs its the easiest possible solution for water to leave
osmosis
the net movement and diffusion of water molecules from a high water potential to a low water potential down the water potential gradient through a partially permeable membrane - passive process
what is a hypertonic solution ?
contains a higher concentration of solutes - has a lower water potential compared to another solution.
what wld happen to rbcs if they were put into a hypertonic solution ?
The rbcs wld lose water through osmosis via the partially permeable membrane
water would diffuse from a high to low water potential.
They would shrink and crenate
what is a hypotonic solution ?
has a lower solute concentration so a higher water potential compared to another solution
what would happen to normal rbcs placed in a hypotonic solution ?
The rbcs wld gain water through osmosis via the partially permeable membrane
water would diffuse from a high to low water potential.
They would expand then eventually rupture and lyse
what is an isotonic solution ?
2 solutions separated by a partially permeable membrane have the same water potential.
There is no net movement of water particles into either solution.
the movement of water into the cell is equal to the movement of water out
why would a rbc eventually burst in distilled water ?
A rbc does not have a cell wall
The influx of water produces a higher (hydrostatic) pressure
the cell membrane expands but cant resist the increasing pressure of the water.
why would a plant cell be less likely to burst when placed in distilled water ?
It has a cell well to resist the pressure exerted by the water and expanding cell membrane
why do plant cells become turgid ?
Plant placed in a solution that has a higher water potential compared to plant cells (hypotonic)
Water diffuses into plant cells via osmosis through a partially permeable membrane.
The water diffuses into the vacuole which expands exerting turgor pressure causing membrane to expand against the cell wall
The plant cell becomes turgid
describe what happens when plant cells are placed in a concentrated salt solution ?
water leaves the cell by osmosis down the water potential gradient through the partially permeable membrane bcs the water potential inside the cells is higher than the salt solutions outside
this causes the causes the cells to become plasmolysed bcs they were placed in a hypertonic solution
plasmolysis
where a plant cell has lost so much water its cytoplasm vacuole and membrane have shrunken away frm the cell wall
what’s the formula for the dilution of the stock concentration ?
vol of stock conc = stock conc required / og conc x vol
precautions when doing dilutions ?
use a new clean micropipette / measuring cylinder for each dilution
ensure adequate mixing at each step
what are the independent and dependent variables in the investigation titled : how does increasing the concentration of a solute (salt) in water effect the mass of potato cubes?
independent - conc of salt
dependent - change in mass
osmosis practical method
cut cylinders from the same part of the same veg using a cork borer- controls conc / water potential
place cylinders next a ruler and cut to the same length (10mm) using a ruler - controls SA
wipe w/ a paper towel to remove excess liquid and measure mass of each cylinder using weighing scales
make up range of diff salt concs and place the same vol of each into separate boiling tubes
add into 25C -optimum-water bath w/ temp controlled -same KE same rate
immerse up to 3 cylinders tissue into each for 30 mins so osmosis can occur
remove cylinders from each solution - blot off excess liquid gently to ensure no overestimation of mass
control variables of osmosis practical
cells from diff veggies will have diff water potentials
time
temp
SA
how are the control variables controlled in osmosis practical ?
use cells frm the same onion
leave onion pieces in solution for sufficient time for osmosis to occur - at least 5 mins
perform exp at same time in a water bath so inion cells r all at the same temp
use a cork borer to ensure diameter is the same and a ruler to cut to same length
what happens if control variables arent controlled in osmosis practical ?
diff water potential gradients e.g. steeper gradients = faster rate
longer time = more osmosis
KE incs = rate of osmosis incs
Greater SA = more particles diffuse at a time = faster rate
osmosis practical precautions
blot potatoes before weighing - no over estimation = no greater % diff
skin potato - skins water proof prevents osmosis
wash and use new piece of apparatus each time - prevents contamination
use thermometer to check temp of water bath -when switching on take time to reach desired temp
same variety potatoes - diff varieties = diff water potential
common improvements in practicals
at least 3 repeats - accurate mean , identify anomalies - carry on for concordant results - improves repeatability
more intermediate values - allows for clearer trend
endocytosis
process of taking material into the cell by means of infoldings or pockets of the cell membrane - usually putting them into a vesicle
how is ATP used in endocytosis
ATP isnt needed for the conformational change of a carrier protein
it is to form the vesicles and fuse w/ the membrane and then to move these vesicles using motor proteins along cytoskeleton threads into the cell interior
exocytosis
a process in which the membrane of the vesicle surrounding the material fuses w/ the cell membrane forcing the contents out the cell
how is ATP used in exocytosis
ATP is needed for the fusion and also to move the motor proteins along the cytoskeleton threads before the fusion to the outside of the cell