transition metals theory
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
73 Terms
formal definition of a transition element
an element that forms at least one stable ion with a part full d-shell of electrons.
why are scandium and zinc d-block elements, but not transition elements?
scandium only forms Sc^(3+)(3d0) in all its compounds, and zinc only forms Zn^(2+)(3d10) in all its compound.
4 bullet point features of transition metals
variable oxidation states, coloured ions, catalytic activity, complex formation
what is meant by the property ‘variable oxidation states’?
transition metals have more than one oxidation state in their compounds. they can thus take part in many redox reactions.
what is meant by the property ‘complex formation’?
transition metals form complex ions. a complex ion is formed when a transition metal ion is surrounded by ions or molecules, collectively called ligands, which are bonded to it by coordinate bonds.
what bonds are involved in the formation of complex ions?
coordinate bonds
what is a ligand
an ion or molecule with a lone pair of electrons that forms a coordinate bond with a transition metal.
what is coordination number
the number of coordinate bonds to ligands that surround the d-block metal ion
what is the normal shape of complexes with coordination number 6?
octahedral
what is the normal shape of complexes with coordination number 4?
square planar or tetrahedral
what are multidentate ligands?
ligands with more than one atom with a loe pair of electrons which can bond to a transition metal ion
name 3 bidentate ligands
ethane-1,2-diamine. ethanedioate. benzene-1,2,-diol
explain the ethane-1,2-diamine ligand (structuralformula, where bonds come from, other names, charge)
NH2CH2CH2NH2. lone pair on each nitrogen. often abbreviated to en. neutral.
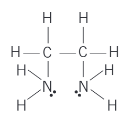
name this molecule
ethane-1,2-diamine or en
explain the ethanedioate ligand (structural formula, where bonds come from, other names, charge)
C2O4^(2-). lone pair on each single bonded oxygen. oxalate. 2-.
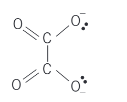
name this molecule
ethanedioate or oxalate
explain the benzene-1,2-diol ligand (formula, where bonds come from, other names, charge)
C6H4(OH)2. lone pair on each oxygen of alcohol groups. 1,2-dihydroxybenzene. neutral.
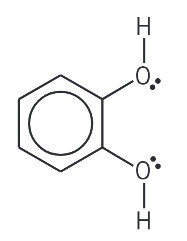
name this molecule
benzene-1,2-diol
name an important multidentate ligand
EDTA^4-
what does EDTA^4- stand for and what is it?
ethylenediaminetetracetate, a hexadentate ligand

name this molecule
EDTA^4-, ethylenediamenetetracetate
where do the coordinate bonds for from with EDTA?
4 single bonded oxygens, 2 nitrogens
what are chelates?
complex ions with polydentate ligands
what can chelates be used for
effectively removing d-block metal ions from solution
what happens if you add a hexadentate ligand to a solution of a transition metal salt?
the EDTA will replace all 6 waterligands in the aqua ion
write the equation for adding EDTA to a solution of a copper salt
[Cu(H2O)6]^(2+)(aq) + EDTA^(4-)(aq) → [CuEDTA]^(2-)(aq) + 6H2O(l)
what is the chelate effect? (not explain)
chelate complexes with polydentate ligands are favoured over complexes with monodentate ligands in the removal of d-block metal ions from solution.
explain the chelate effect:
using a multidentate ligand (instead of a monodentate ligand) means there will be a greater number of product particles than reactant particles. this means there will be an increase in entropy which drives the reactant to the right.
what can bi/multidentate ligands do instead of bonding to the same metal to form a ring
the ligand sites can bond to two different metal ions and act as a bridge
why are most transition metal compounds coloured? (6 sentences)
transition metal compounds have part-filled d-orbitals. it is therefore possible for electrons to move from one d-orbital to another. in an isolated transition metal atom, all the d-orbitals are of exactly the same energy, but in a compound, the presence of other atoms means the d-orbitals have slightly different energies. when electrons move from one d-orbital to another of a higher energy they absorb energy in the visible region of the spectrum equal to the difference in energy between levels. this colour is therefore missing from the spectrum. observed is a combination of all the colours not absorbed.
what is the equation relating energy, frequency, and Planck’s constant?
delta E = hv = hc/lambda
violet light is of what kind of energy and frequency
high energy, high frequency
red light is what kind of energy and frequecny
low energy, low frequency
what does delta E depend on?
the oxidation state of the metal, the ligand, the complex shape
how can the frequencies a complex absorbs be measured
a UVvisible spectrometer
what is the relationship between uv/vis light and concentration
the more concentrated the solution, the more light absorbed
what can be added to intensify the colours of some ions
ligands, e.g. SCN^-
explain the variable oxidation states used in Tollen’s
as the aldehyde is oxidised to a carboxylic acid, the colourless Ag(+1) in [Ag(NH3)2]^+ is reduced to Ag(0) in silver mirror
explain the variable oxidation states used in Fehlings
as the aldehyde is oxidised to a carboxylic acid, the blue Cu^(+2) is reduced to brick red Cu(+1) in Cu2O
explain the variable oxidation states in K2Cr2O7
as alcohol/aldehyde is oxidised, the orange Cr(+6) in Cr2O7^(2-) is reduced to green Cr(3+)
what affects how easy it is to change the oxidation state of a transition metal?
the pH and the ligands present
in general, is it easier to oxidise or reduce a transition metal in alkaline conditions?
oxidise
in general, is it easier to oxidise or reduce a transition metal in acidic conditions?
reduce
how can you show that it is easier to reduce a transition metal in acidic conditions?
using electrode potentials
write the half equation for the reduction of Mn(VII) to Mn(II)
MnO4^-(aq) + 5e^- + 8H^+(aq) → Mn^(2+)(aq) + 4H2O(l)
write the half equation for Fe(II) to Fe(III)
Fe^(2+)(aq) → Fe^(3+)(aq) + e^-
write the full ionic equation for the oxidation of iron (II) to iron (III) using potassium manganate (VII)
5Fe^(2+)(aq) + MnO4^-(aq) + 8H^+(aq) →5Fe^(3+)(aq) + Mn^(2+)(aq)+ 4H2O(l)
what is the colour change for the oxidation of iron (II) to iron (III) using potassium manganate (VII)
deep purple to colourless
colour of Fe^(2+)
pale green
colour of MnO4^-
intense purple
colour of Fe^(3+)
pale violet
colour of Mn^(2+)
pale pink
what conditions should the oxidation of Iron (II) to Iron (III) using potassium mangate be done under
acidic
which acid should be used for the oxidation of Iron (II) to Iron (III) using potassium mangate
sulfuric acid
why should sulfuric acid be used in the oxidation of Iron (II) to Iron (III) using potassium mangate instead of hydrochloric?
E standard values show that manganate ions will oxidise chloride ions to chlorine (the emf value is positive). this would affect the titration because the manganate ions must be used only to oxidise Fe^(2+) ions. manganate ions do not oxidise sulfate ions.
what are heterogeneous catalysts
catalysts present in a reaction in a different phase than the reactants
what state are heterogeneous catalysts usually present as?
solids
give two ways to make heterogeneous
increase their surface area. spread the catalyst onto an inert support medium.
what is poisoning
the surface if the catalyst becoming covered with unwanted impurities.
why does spreading the catalyst onto an inert support medium make heterogeneous catalysts more efficient?
it increases the surface:mass ratio
what is the catalyst for the haber process?
iron in pea sized lumps
how long does the iron catalyst last for in the haber process? what happens to it?
around 5 years. it becomes poisoned by impurities in the gas stream like sulfur compounds
what is produced from the Contact Process
sulfuric acid
what is a homogeneous catalyst
when the catalyst is in the same phase as the reactant.
what happens when a homogeneous catalyst is used (and not a heterogeneous one)
an intemediate species is formed
write the overall ionic equation for peroxodisulfate ions oxidising iodide ions to iodine
S2O8^(2-)(aq) + 2I^-(aq) → 2SO4^(2-)(aq) + I2(aq)
what catalyses the reactopm betweem peroxodisulfate ions oxidising iodide ions to iodine?
Fe^(3+) ions
write both steps of the catalysed redox reaction between peroxodisulfate ions and iodide ions (catalysed by iron(II) ions
1) S2O8^(2-)(aq) + 2Fe^(2+)(aq) → 2SO4^(2-)(aq) + 2Fe^(3+)(aq). 2) 2Fe^(3+)(aq) + 2I^-(aq) → 2Fe^(2+)(aq) + I2(aq)
why does the uncatalysed reaction between peroxodisulfate ions and iodide ions have such high activation energy?
it takes place between two ions of the same negative charge, which repel, so it has a high activation energy.
give an example of an autocatalysed reaction. what is the catalyst?
ethanedioic acid + potassium manganate (VII). Mn^(2+)
which titration is used to find the concentration of potassium manganate (VII) in solution?
titration of ethanedioic acid and potassium manganate
write the ionic equation for potassium manganate + ethanedioic acid
2MnO4^-(aq) + 16H^+(aq) + 5C2O4^(2-)(aq) → 2Mn^(2+)(aq) + 8H2O(l) + 10CO2(g)