DF2-
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
What does Hess’ Law State
The enthalpy change for any reaction is independent of the intermediate stages as long as the initial and final conditions are the same for each route.
Equation for enthalpy cycle of combustion? & reactants
H1= H2 - H3
H2 =reactions
H3= products
(Same for reactants-equation)
Combustion products are always carbon dioxide and water, you add the molecules that are missing with the arrows (adding oxygen)
Remember that the - is already in the equation and any other signs of the reactants or products are added on
Equation for enthalpy change of formation?
H1= -H2 + H3
H2 = reactants
H3 = products
Splitting all of the reactants/products into their individual elements eg: N2H4 into N2 and 2H2
Remember that the - sign isn’t removed and that if my numbers value is negative it becomes positive
Key things to remember about enthalpy change of reaction?
the diatomic molecules are split into elements with the number of them in front of the element
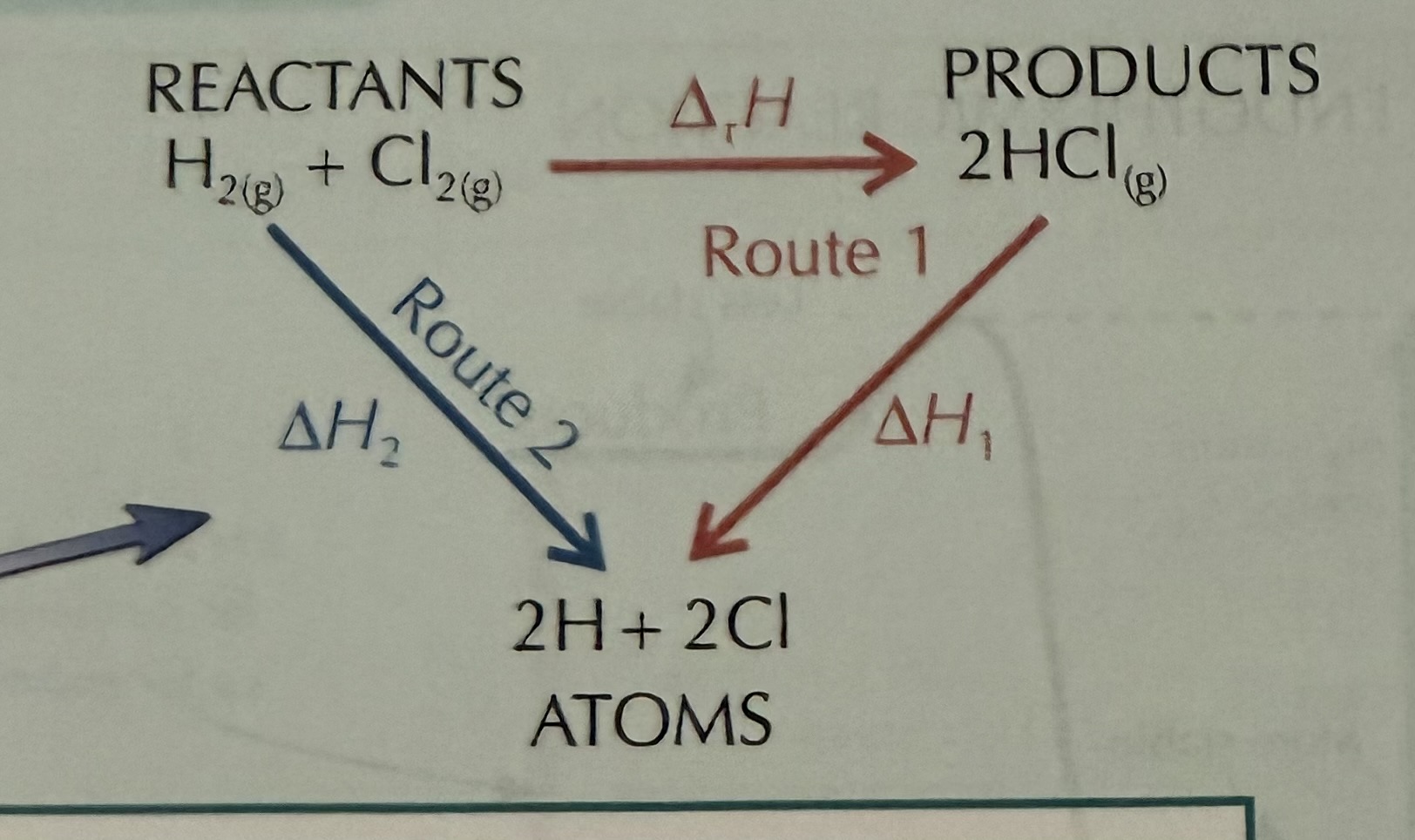
What direction do arrows point in combustion? (& reactants)
Down
What direction do arrows point in formation?
Up