Unit 5 past paper questions.
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
The platinum electrode in the standard hydrogen electrode is coated in platinum black. Suggest why platinum is used as the electrode and why it is coated in platinum black (2)
Platinum is an inert metal so it does not take part in the reaction.
Platinum black increases the surface area of the electrode to allow closer contact of hydrogen ions and H+ ions in solution.
Explain why a reference electrode is needed to measure the electrode potentials of chemical systems
A potential difference requires a complete circuit with two electrodes because it is measured between two points. The potential difference needs to be measured relative to the reference point
Sometimes the calculates Ecell value shows it to be thermodynamically feasible but in the laboratory no reaction occurs. Give 2 possible reasons why
Activation energy is very high and cannot be overcome
Conditions are not standard (298k, 1 mol/dm3)
How do you calculate overall equations from electrode reaction equations?
Pick the two equations with the desired compounds
Flip the equation according to the reactant and product order in the question
Balance the number of electrons if needed and cancel out like terms on the other side of the equation




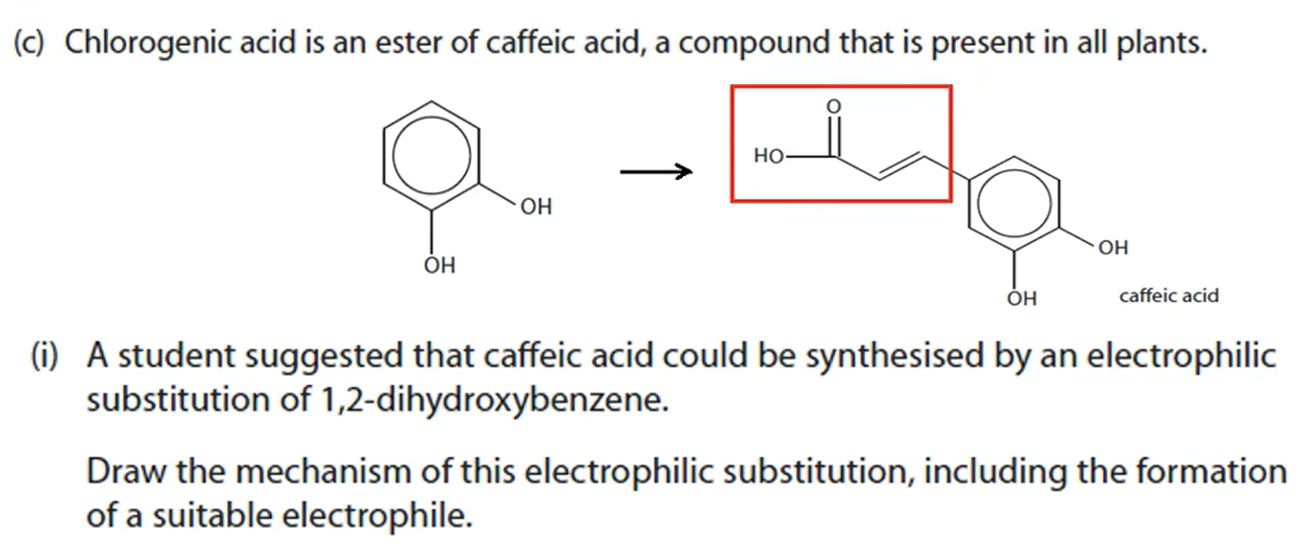
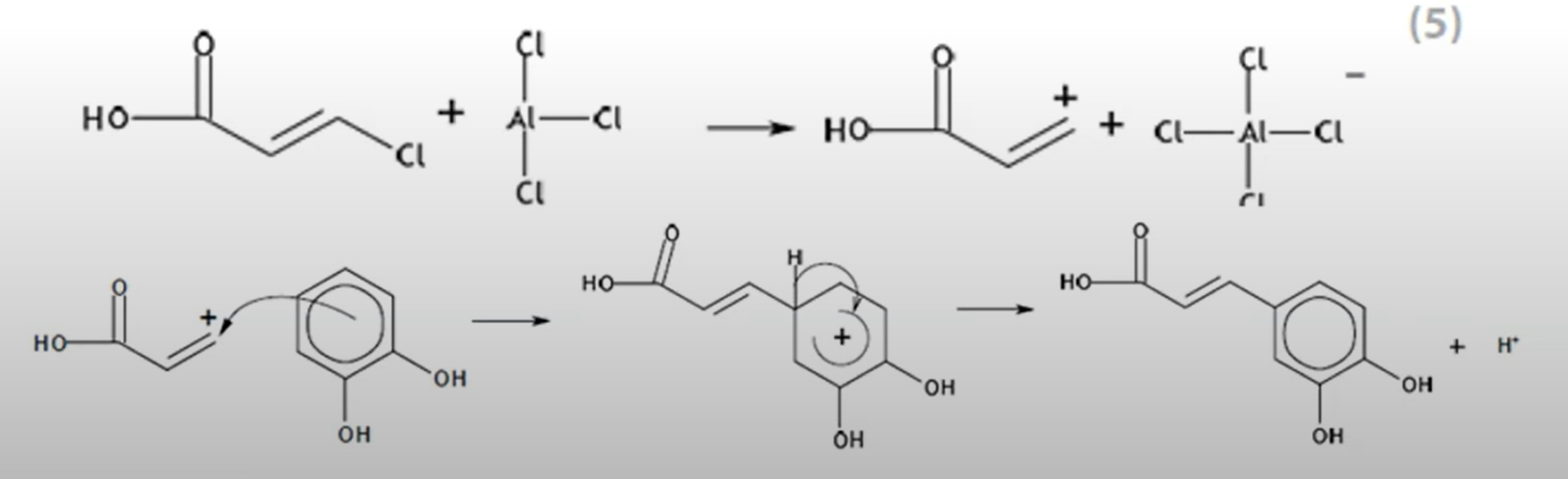
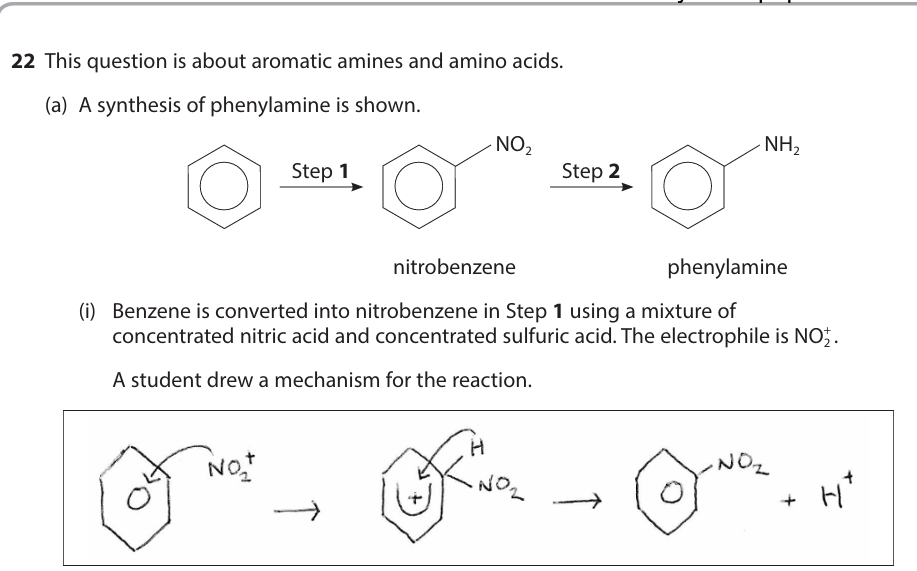


Both act as catalysts by providing an alternative pathway with lower activation energy. They therefore speed up the rate of reaction since a greater fraction of molecules will have energy greater than the activation energy, so more successful collisions occur.
For the differences, heterogenous catalysts are in a different phase than the reactants while homogenous catalysts are in the same phase
Examples of heterogenous catalysts: iron in the haber process, nickel in hydrogenation of alkenes and platinum in catalytic converters
Exaples of homogenous catalysts include Fe2+ or Fe3+ in the reaction between iodide and persulfate ions
During mechanisms involving heterogenous catalysts, reactant molecules are adsorbed on the surface of the catalysts. The bonds between the reactant molecules are weakened, causing a reaction to take place on the catalyst surface to form products, which are then desorbed from the surface of the catalysts.
During mechanisms involving homogenous catalysts, a transition metal is oxidised/reduced to a different oxidation state in one step. The oxidised/reduced version is then changed back to it’s original state in the next step of the reaction mechanism.


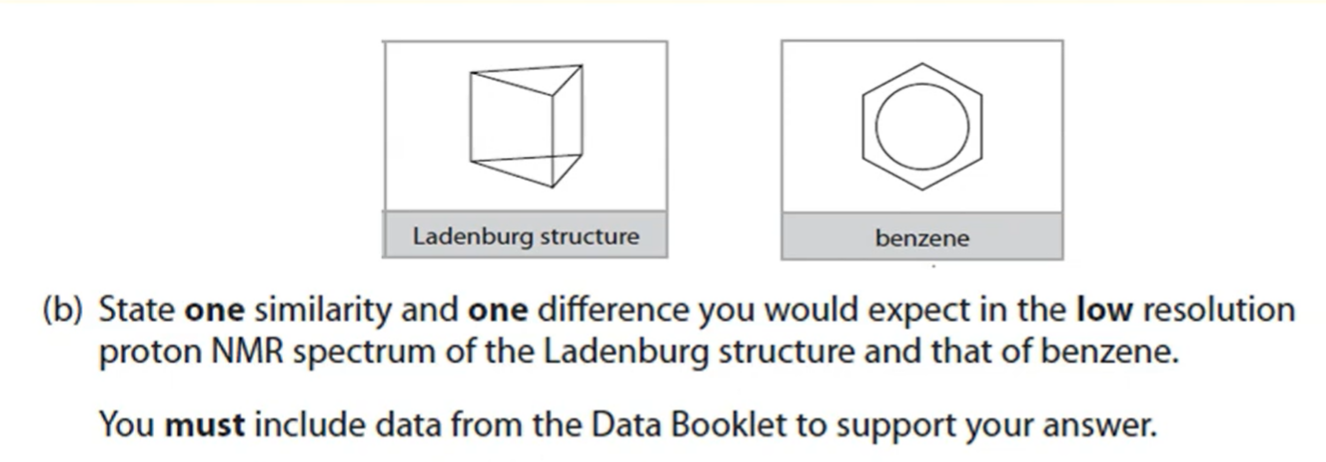




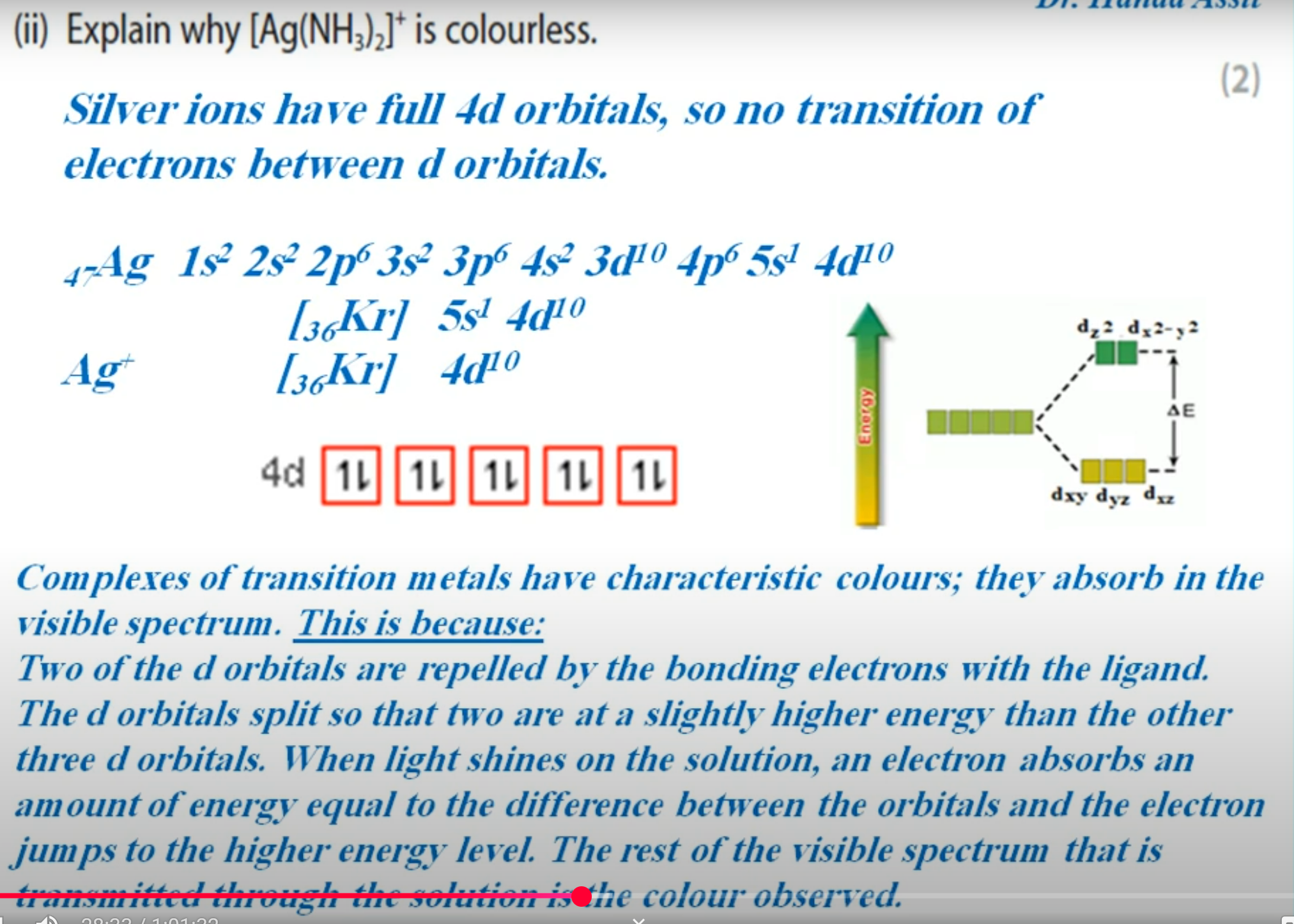

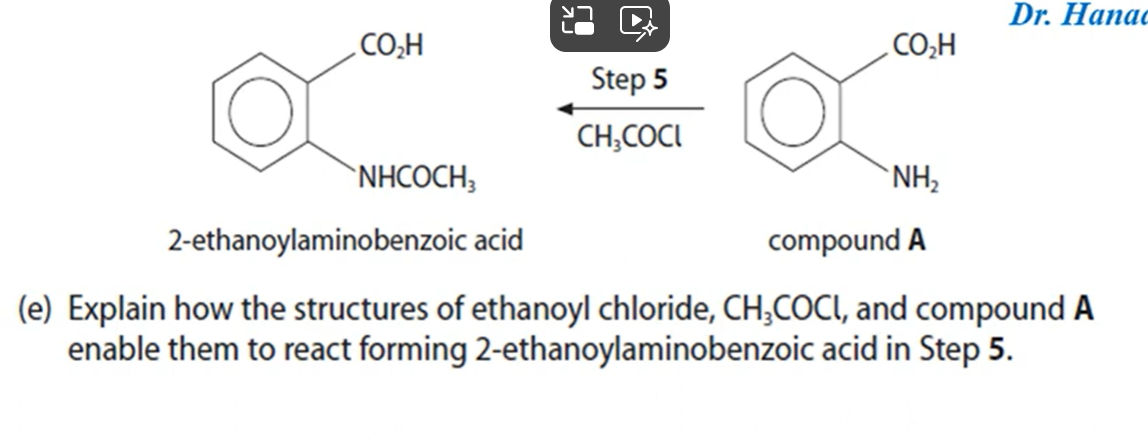



Explain why some ligand complexes are coloured while others are not (3 marks)
Colors are a result of electron transitions between split 3d orbitals
Some transition metals have partially filled orbitals so transitions are possible
Other transition metals have full 3d orbitals so no electron transitions can occur.
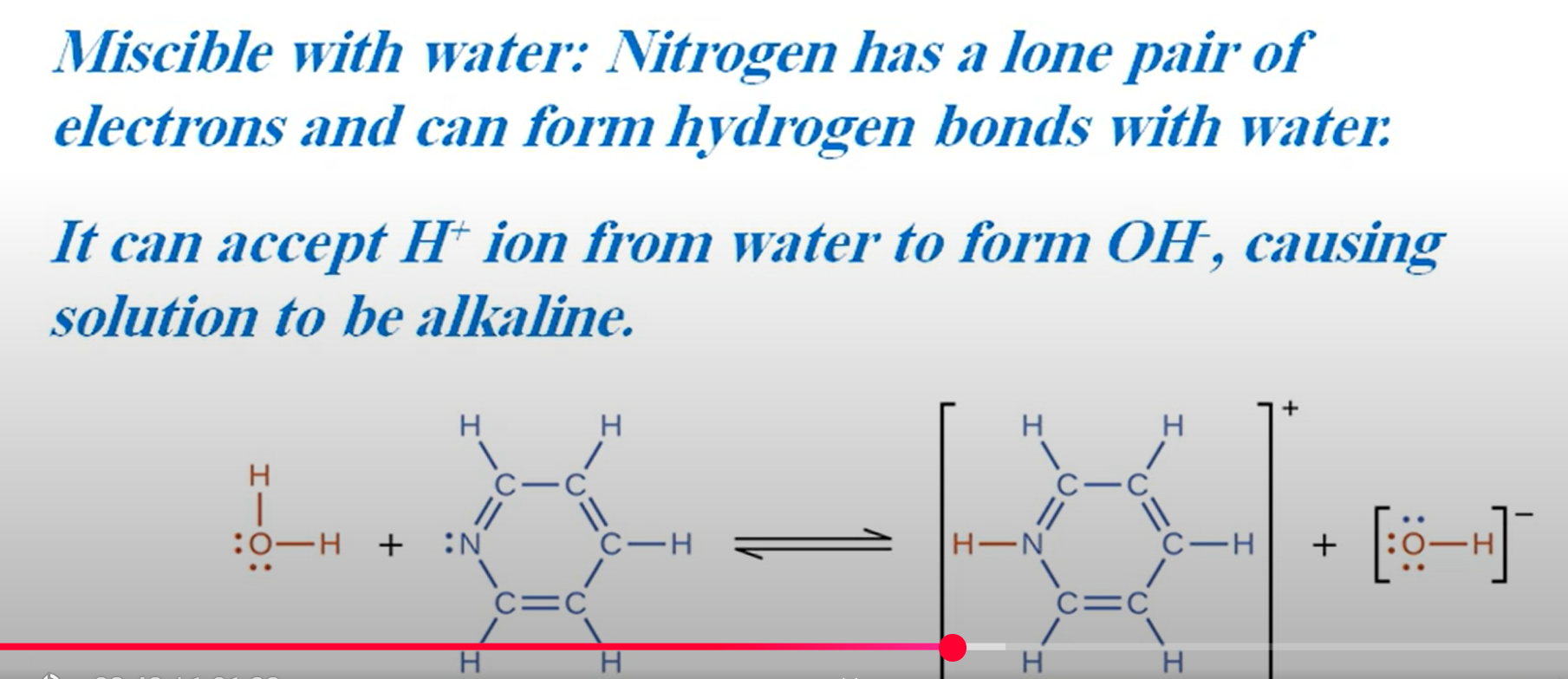
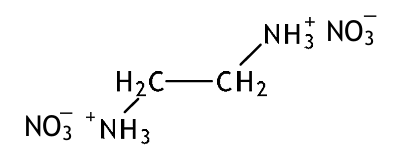
When nitric acid was added to ethane-1,2-diamine and the resulting solution allowed to evaporate to dryness at room temperature, white crystals remained. Explain the chemical reaction that occurred and give the structure of the white crystals (3 marks)
This is a neutralisation reaction and ethane-1,2-diamine acts as a base.
This is because of the lone pairs on the nitrogen atoms that allow them to accept protons
Ethane-1,2-diamine reacts to form a salt.

V+5 has high charge density
So it will polarise the h2o molecule
This can lead to deprotonation, so [V(H2O)6]5+ cannot form