Microbiology Labs Part 2
1/60
Earn XP
Description and Tags
Second half of the microbiology labs: Plaque Assay Standard Plate Count Transformation ELISA
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
Topic: Plaque Assay of Virus Titer (section 10)
What is a countable plate
plate that has 30 to 300 colonies
What are bacteriophages
viruses that infect bacteria
What bacteriophage do we use to perform the plaque assay?
T4 coliphage
Lytic replication
aka lytic cycle
5 steps
What are the 5 steps of the lytic cycle?
Attachment
Penetration
Synthesis
Assembly
Release
Describe attachment
phages attach to the host bacterial cell
host bacterial cell - E.coli
Describe penetration
the phage injects its DNA into E.coli
Describe synthesis
phage’s DNA directs protein synthesis to make the various parts of the phage
each replicate phage needs a copy of original phage’s genome
the bacteriophage DNA is also copied (replicated)
What is a genome
the complete set of genes or genetic material present in a cell or organism
Describe assembly
the second generation of phages are built
capsid subunits come together to form the capsid into which the phage genome is inserted
tail fibers come together and attach to the capsid
the host bacterium's DNA is degraded into short segments.
Why is it important for the host bacterium’s DNA to be degraded into short segments?
It paves the way for host cell DNA to be transferred to a new bacterial host using generalized transduction
What is generalized transduction?
the process by which phages can package any bacterial DNA and transfer it to another bacterium.
Describe release
The host bacterial cells burst (lyses) releasing the progeny phages, which begin the replicative cycle all over again.
How long does the entire T4 coliphage lytic cycle take?
25 minutes and releases a few 100 secondary phages each time
What is a plaque?
a clearing that results from the lysis of bacterial cells growing in a lawn on an agar plate
used to calculate phage concentration (titer)
What is plaque assay
determines the concentration and morphology of bacteriophages
Similarities between plaque assay and standard plate count
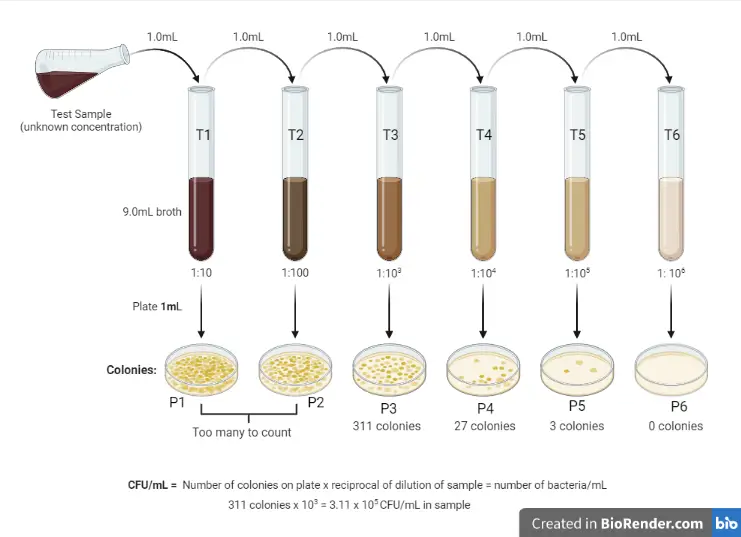
Both use serial dilution to produce countable plates
Difference between plaque assay and standard plate count
the plaque assay is done using the pour-plate technique
bacterial cells and viruses are first added to molten agar and then poured into the plate.
Why is soft agar used?
Its’ consistency is sufficient to immobilize the bacteria while allowing the smaller bacteriophages to diffuse short distances and infect surrounding cells.
Formula for phage titer
OCD = pfu/original sample volume or pfu/(dilution x volume)
What is the function of serial dilution
used to decrease a bacterial concentration to a required concentration for a specific test method, or to a concentration which is easier to count when plated to an agar plate
A visible plaque on a lawn of bacteria grown on a plate indicates
where phage has replicated in bacteria resulting in bacterial lysis
In the plaque assay of virus titer, the 15-minute pre-absorption period functions to
allow phage to attach to bacterial cells.
In the plaque assay of virus titer, how does the volume of the bacteria used factor into the dilution factor of the phage sample?
It does not factor in since the whole volume of cells is plated.
Process of plaque assay (specific)
Obtain Materials and Label Samples
Label the seven sterile test tubes 1 through 7
Label the seven microtubes E. coli 1–7.
Label the TSA (or nutrient agar) plates A through G. Place them in the 35°C incubator to warm them. Take them out one at a time when called for in the procedure.
Dilute the phage sample
Aseptically transfer 9.9 mL sterile normal saline to dilution tube 1.
Aseptically transfer 9.0 mL sterile normal saline to dilution tubes 2–7.
Mix the T4 suspension and aseptically transfer 0.1 mL to dilution tube 1 and mix well. This is a 10-2 dilution.
Then, aseptically transfer 1.0 mL from dilution tube 1 to dilution tube 2 and mix well. This is a 10-3 dilution.
Now, aseptically transfer 1.0 mL from dilution tube 2 to dilution tube 3 and mix well. This is a 10–4 dilution.
Continue in this manner through dilution tube 7. The dilution of tube 7 should be 10-8.
Mix E. coli with phage dilutions
Mix the E. coli culture and aseptically transfer 0.3 mL into each of the seven E. coli tubes that you labeled in Step 1
Aseptically transfer 0.1 mL sterile normal saline to E. coli tube 1. This will be mixed with 2.5 mL soft agar and used to inoculate a control plate.
Aseptically transfer 0.1 mL from dilution tube 2 to its companion E. coli tube. Repeat this procedure with the remaining five tubes.
Allow samples to stand undisturbed for 15 minutes
Add soft agar to E. coli tube 1
Use a sterile transfer pipette to add the entire contents of E. coli tube 1 to the soft agar. Mix well with the pipette and immediately transfer it onto plate A.
Label the plate “Control.”
Add soft agar to E. coli tube 2
Remove a second soft agar tube from the water bath and add the entire contents of E. coli tube 2 with a sterile transfer pipette.
Label it 10–410–4 mL (this is the phage sample volume).
Add soft agar to E. coli tubes 3-7
Repeat the agar addition procedure with dilutions 10–410–4 through 10–810–8 and plates C through G.
Label the plates with the appropriate sample volume.
Allow plates to solidify
Incubate
Make and record observations
Calculate the original phage titer
Process of plaque assay (simple)
Obtain Materials and Label Samples
Dilute the phage sample
Mix E. coli with phage dilutions
Allow samples to stand undisturbed for 15 minutes
Add soft agar to E. coli tube 1
Add soft agar to E. coli tube 2
Add soft agar to E. coli tubes 3-7
Allow plates to solidify
Incubate
Make and record observations
Calculate the original phage titer
Double layer agar technology
agar overlay
Why is it necessary to grow the bacteria in the plaque assay as a lawn?
It allows for visualization of plaques.
The water bath that was used to keep the soft agar warm in the plaque assay was set to 80°C instead of the recommended 50°C. What is the most likely consequence(s)?
The agar would be too hot and could kill the E. coli when added.
The agar would be too hot and could inactivate the phages when added.
Consider Plate A (inoculated with only E. coli) of the plaque assay of virus titer. What best describes this plate's function?
It served as a negative control.
What would the calculated original phage titer be for the following data?
Phage dilution tube: 0.2 mL of a 10-4 dilution; E. coli: 300 μL; soft agar: 2.5 mL; 377 plaques observed
18,850,000 PFU/mL
What would the calculated original phage titer be for the following data?
Phage dilution tube: 0.1 mL of a 10-9 dilution; E. coli 500 μL; soft agar: 2.5 mL; 216 plaques observed
2.16 x 1012 PFU/mL
Formula for dilution factor
D0V0=D1V1
Standard Plate Count (topic)
What is the function of the control plate?
checks for viability of bacteria
contains no phage
should resemble bacterial lawn
Transformation Lab (topic)
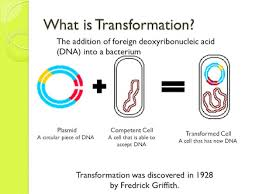
What is the definition of transformation
Is the procedure in which bacteria with recipient cells acquires foreign DNA from the environment
Procedure (15)
Label tubes. One will be +plasmid”. The other will be “-plasmid”.
Add 250 microliters of calcium chloride to each tube
Place both tubes on ice
Transfer E.coli bacteria from starter plate to the +plasmid tube
Suspend the cells by pipetting in and out with a sterile transfer pipette
Return the +plasmid tube to ice
Transfer E.coli bacteria from starter plate to the -plasmid tube.
Repeat steps 4 and 5.
Return -plasmid tube to ice
Use sterile disposable inoculating loop to add 10 microliters of plasmid DNA to +plasmid tube ONLY.
Put the loopful of plasmid DNA directly into the cell suspension and spin the loops to mix the DNA with the cell
Return +plasmid tube to ice and incubate both tubes on ice for 15 minutes
Label media plates
After 15-min incubation on ice, heat shock.
Place tubes in 42 C water bath for 90 secs and gently agitate the tubes while waiting.
Return both tubes to ice for 1 minute
Add 250 microliters of LB broth to each tube
Allow tubes to sit for 5-15 minutes for incubation
Add 5 glass beads
Add 100 microliters of cells from +plasmid tube onto +plasmid plates
Repeat for -plasmid tube
Sit for several minutes
Incubate overnight
Why do we add calcium chloride?
Weakens cell membrane permeability
Makes the cell more competent
Why do we manipulate the temperatures?
heat shock - creates pores in the plasma membrane of the bacteria and allows for plasmid DNA to enter the bacterial cell.
ice bath - aids in the better attachment of plasmid DNA to the bacterial cell membrane
Why do we add LB broth?
It provides nutrient composition, concentration, and culture conditions needed for bacteria to grow
Major components of this lab
Calcium chloride
Plasmid DNA
Bacteria
Temperature Changes
Expected results (LB/KAN)
LB/amp+plasmid plate
No lawn, no colonies
LB/amp-plasmid plate
No lawn, no colonies
LB/kan+plasmid plate
colonies
LB/kan-plasmid plate
No lawn, no colonies
LB+plasmid
bacterial lawn
LB-plasmid
bacterial lawn
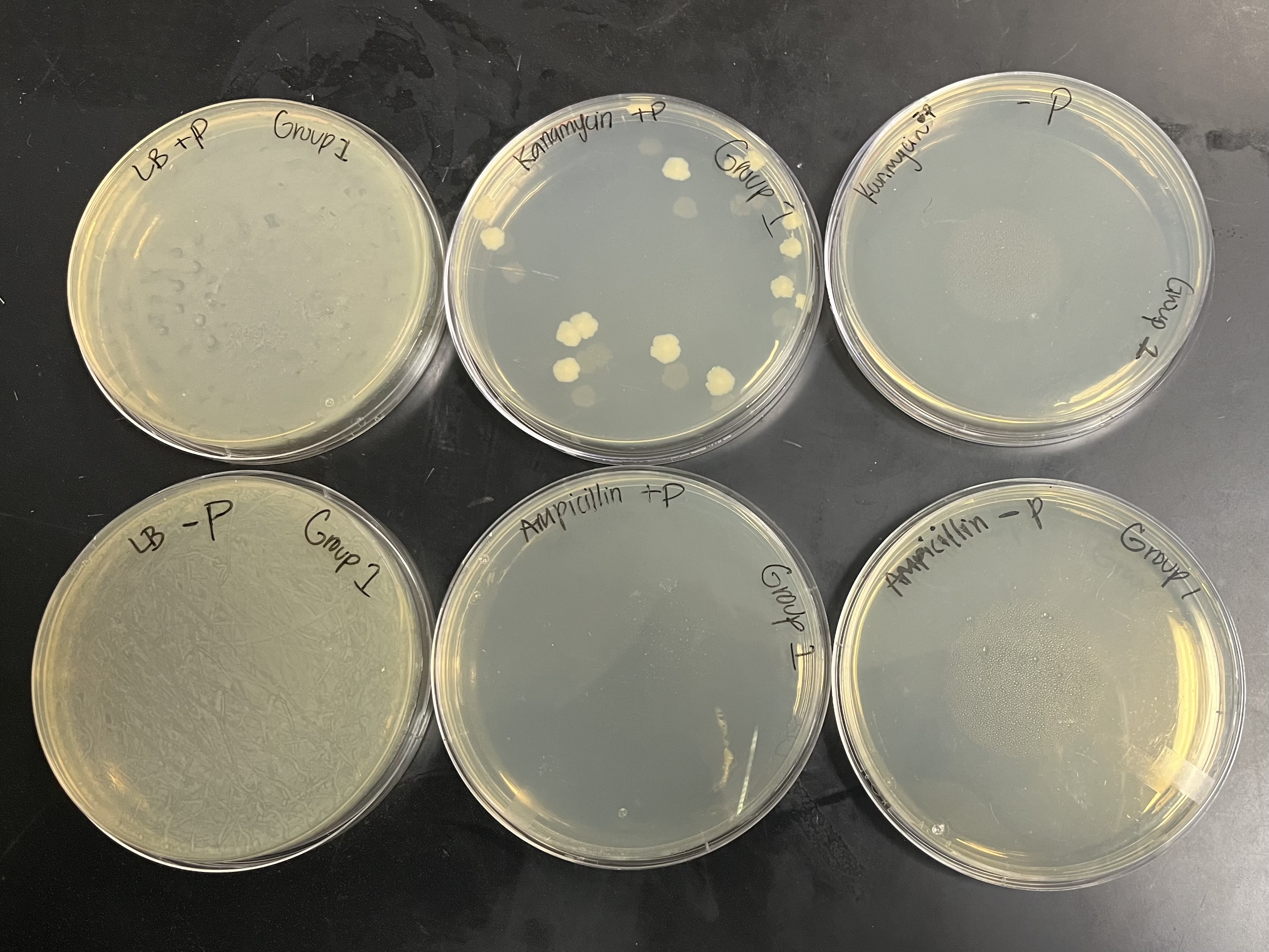
Why is there growth on the LB/plasmid plates?
bacterial lawn
no antibiotic was present in the LB plate, so all the cells could grow, even those without a plasmid
Why is there growth on the LB/kan+plasmid plate?
growth occurs in presence of kanamycin (resistant)
Why is there no growth on the LB/kan-plasmid plate?
no plasmid introduced to bacteria
it is not resistant
it is sensitive to kanamycin so there is no growth
What is there no growth on the LB/amp plates?
No selective pressure
Should you plate some of your transformed bacteria onto plates with antibiotics? Why or why not?
Yes, you should plate some of your transformed bacteria onto plates with antibiotics. This step is useful because you need to determine which bacteria are resistant to antibiotics and which are not.
How will you differentiate between bacteria that have taken up the plasmid and those that have not?
We can use selection markers to determine which bacteria has taken up the plasmid and which has not.
After transforming bacteria with the plasmid, you can plate the bacteria on agar plates containing the antibiotic.
Only bacteria that have successfully taken up the plasmid with the resistance gene will survive and grow on these plates, indicating transformation.
Reporter genes
green fluorescent protein (GFP) gene.
Positive control
Bacteria transformed with a plasmid known to confer a specific phenotype (e.g., antibiotic resistance or fluorescence) should display the expected phenotype.
Negative control
no plasmid
no growth on plates containing antibiotic
ELISA (topic)
What is ELISA
enzyme-linked immunosorbent assay
What is the purpose of ELISA
this process used antibodies to detect the presence of a disease agent in your blood or bodily fluid
Clinical application of ELISA
can be used in pregnancy tests, COVID tests, HIV tests, etc
ELISA Procedure
Load bodily fluid
Antigen or nonantigen binds via phosphorate (nonspecific binding to surface)
Wash 3 times to remove nonbinding particles
Add primary antibody
Wash 3 times
If antigen is present, primary antibody will stay. If antigen is not present, antibody will be washed away
Add secondary antibody
Wash 3 times
Transfer 50 microliters of enzyme substrate into all 12 wells
If disease is present, the color turns blue
Why do we need triplets for each test?
for accuracy
Why do we need positive and negative controls for ELISA?
they test the viability of the experiment or of the antigen and primary antibody