Hydrogen Fuel Cells
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
A diagram of the hydrogen fuel cell.
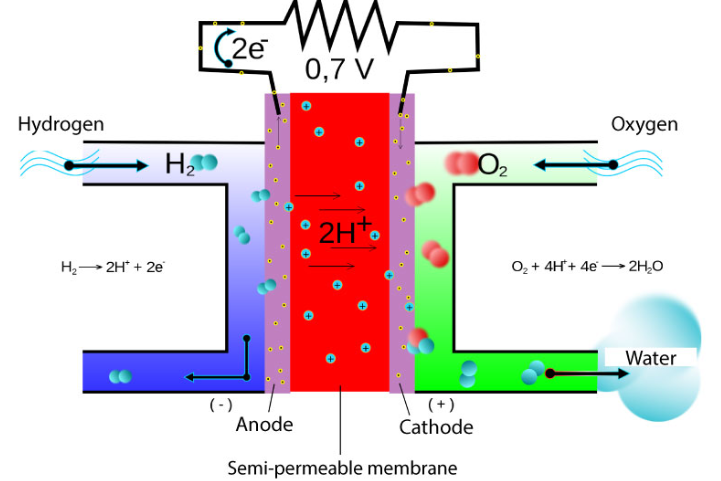
What redox process occurs at the anode?
At the anode, hydrogen gas is oxidised to form H+ ions:
H2→2H++2e−
What redox process occurs at the cathode?
At the cathode, oxygen gas is reduced to water:
O2 + 4H+ + 4e−→2H2O
What is the overall reaction of a hydrogen fuel cell?
O2 + 2H2→2H2O
What are the advantages of using hydrogen fuel cells?
Water is the only waste product, so hydrogen fuel cells do not contribute to global warming by releasing carbon dioxide or other greenhouse gases.
Far less energy is lost as heat, making hydrogen fuel cells far more efficient.
Hydrogen gas can be produced by the electrolysis of water, making it a renewable energy source.
What are the disadvantages of using hydrogen fuel cells?
Hydrogen gas is highly flammable. Storing gases under pressure is difficult enough but extra precautions must be taken to store flammable gases, making hydrogen a difficult and expensive fuel to store.
The most common source of hydrogen is the reformation of methane from fossil fuels. This means that crude oil is still being used and carbon dioxide is still being formed when hydrogen is used, just at an earlier stage of production.
Producing hydrogen by electrolysis requires a lot of electricity, which means that producing hydrogen releases carbon dioxide unless the electricity is produced from renewable sources.