carboxylic acids
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
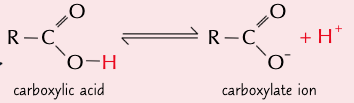
give the eqn for the partial dissociation of carboxylic acids in water:
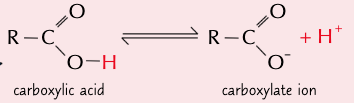
state where the PoE lies for the partial dissociation of carboxylic acids in water and explain why:
PoE lies to the left as most molecules do not dissociate
∴ making carboxylic acids weak acids
give the general word eqn for the reaction between an acid and alkali:
acid + alkali → salt + water
give the general word eqn for the reaction between an acid and a carbonate:
acid + carbonate → carbon dioxide + salt + water
give the general word eqn for the reaction between an acid and a metal:
acid + metal → salt + hydrogen
give the eqn for the reaction between ethanoic acid and sodium hydroxide - name the product:
CH3COOH + NaOH → CH3COO-Na+ + H2O
CH3COO-Na+ = sodium ethanoate (this is a salt, not an ester!)
give the eqn for the reaction between ethanoic acid and ammonia - name the product:
CH3COOH + NH3 → CH3COO-NH4+
ammonium ethanoate (this is a salt, not an ester!)
give the eqn for the reaction between ethanoic acid and sodium carbonate and name the product:
2CH3COOH + Na2CO3 → CO2 + H2O + 2CH3COO-Na+
2CH3COO-Na+ = sodium ethanoate
give the eqn for the reaction between ethanoic acid and sodium and name the product:
2CH3COOH + 2Na → H2 + 2CH3COO-Na+
2CH3COO-Na+ = sodium ethanoate
give the eqn for the reaction between ethanoic acid and magnesium and name the product:
2CH3COOH + Mg → H2 + (2CH3COO-)2Mg2+
(2CH3COO-)2Mg2+ = magnesium ethanoate