Chapter 8 (part 1):enzyme types, reaction rates, lysozyme, and chymotrypsin
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
83 Terms
Enzyme
proteins that catalyze reactions. Biological catalysts
Enzymes catalyze thermodynamically —-- reactions causing them to proceed at —- rates
favorable; rapid
Enzymes provides cell with the ability to exert —--- over —---
kinetic control over thermodynamic potentiality
Living systems use enzymes to —- and —-- the rates of vitally important biochemical reactions
accelerate and control
Enzymes are the agents of —- function
metabolic
t/f virtually all reactions in cells are mediated by enzymes
true
Ribozyme
RNA that catalyzes reactions. (only difference from enzymes is that enzymes are proteins)
Enzymes catalyze (stereo-selective or stereo-specific) biochemical reactions
stereo-selective
Specificity
term used to define the selectivity of enzymes for their substrates
Catalytic power
the ratio of enzyme-catalyzed rate of a reaction to the uncatalyzed rate
t/f enzymes are able to change the overall free energy of a reaction
FALSE
t/f enzymes are able to change the Keq of a reaction
FALSE
—- of enzyme activities ensure that rate of metabolic reactions is appropriate to cellular requirements
regulation
Enzymes are —- and —-- sensitive
pH and temperature
Oxioreductases
enzyme that catalyzes redox reactions
Transferases
enzyme that catalyzes transfer of a functional group (like a methyl group or phosphate)
Hydrolases
enzyme that catalyzes hydrolysis reaction, which breaks a chemical bond by the addition of a water molecule
Lyases
enzyme that catalyzes the cleavage of carbon-carbon or carbon-heteroatom bonds, such as C-O, C-N, and C-S, by an elimination mechanism that is not hydrolysis or oxidation
Isomerases
enzyme that catalyzes intramolecular rearrangements, converting one isomer into another
Ligases
enzyme that catalyzes joining of two molecules together, often with the energy from ATP hydrolysis
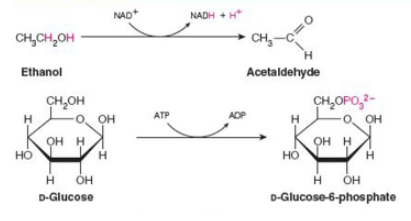
what class of enzyme is catalyzing each reaction shown?
top-oxidoreductase; bottom-transferase
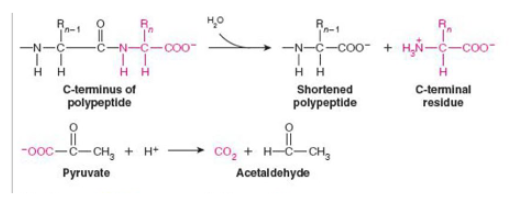
what class of enzyme is catalyzing each reaction shown?
top-hydrolyase ;bottom-lyase
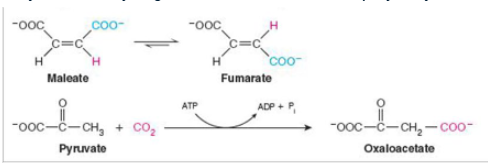
what class of enzyme is catalyzing each reaction shown?
top-isomerase ;bottom-ligase
k
rate constant. quantifies the speed of a chemical reaction by relating the reaction rate to the concentrations of reactants. A higher rate constant indicates a faster reaction, while a lower value means a slower reaction
1st order reaction
rate is dependent on the concentration of only one reagent
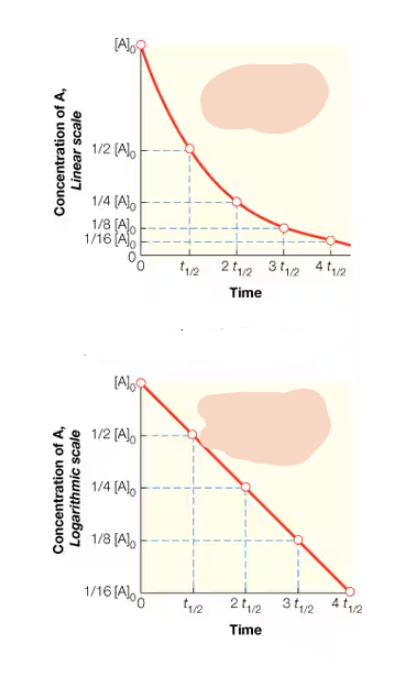
what order rxn is this? How can the K-value of this rxn. Be deduced?
1st order
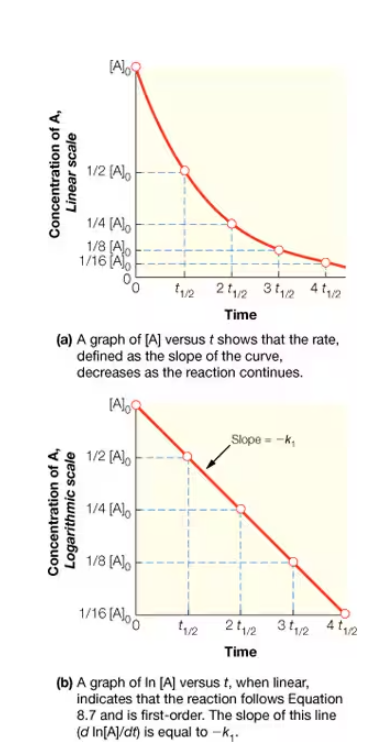
0th order reaction
rate is independent of concentration of reagent
Example of 0th order reaction
isotope decay
2nd order reaction
rate is dependent on the concentration of two reagents
For the reaction A→B, what order of reaction is this?
1st order
Show the equation for reaction rate of a 1st order reaction (A→B)
(n=1 for a 1st order rxn.)
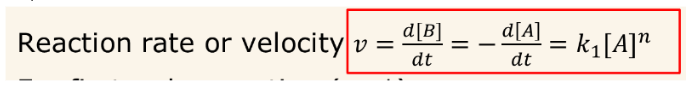
For the 1 order reaction A→ B, how could you find the concentration of A at a certain time t?
A+B→ C, what order of reaction is this?
2nd order
Show the equation for reaction rate of a 2nd order reaction (A+B→ C)
v= k1 [A]^n [B]^m
Binding of substrate to enzyme is usually what order of rxn?
second order
Every object will stay at its (lowest or highest) energy state if —--
lowest; there is a path
Every molecule exists at its —--- energy —--. To visit a lower —-, what must it do?
Local energy minimum; to visit a lower minimum, it must go over the energy barrier
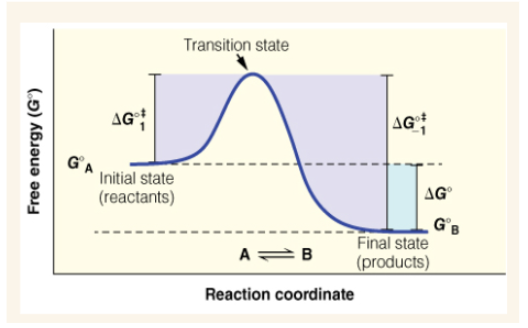
Free energy diagram (y axis and x axis)
y axis is free energy (G naught) and x-axis is reaction coordinate
Equation for standard state free energy change of a reaction
∆Gº = ∆GºA-∆GºB
∆Gºǂ1 (delta G naught double dagger)
free energy of activation of the reaction
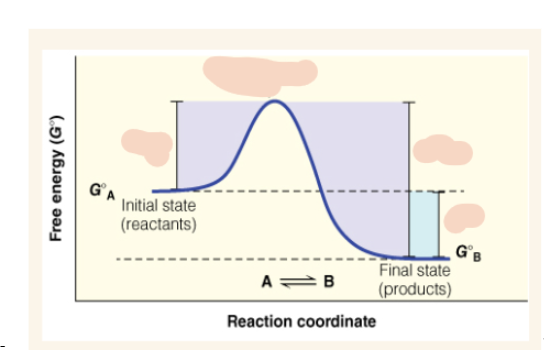
What is the ∆Gº and ∆Gºǂ1 of this reaction?
Arrhenius equation
A=Arrhenius constant
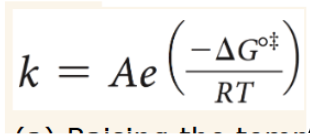
R=?
8.314 J/(K*mol)
Raising temperature (T) increases or decreases rate constant (k)
increases
Raising ∆Gºǂ increases or decreases rate constant (k)
decreases
∆∆Gºǂ=?
∆Gºǂnon - ∆Gºǂcat (difference in activation energies between noncatalyzed and cata;yzed reactions)
Enzymes preferentially stabilize the —- of a reaction
transition state
Enzymes create favorable free energy due to favorable —--
bonding enthalpy (∆H)
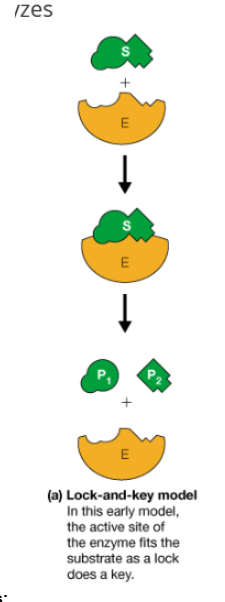
Lock and key model of enzymes
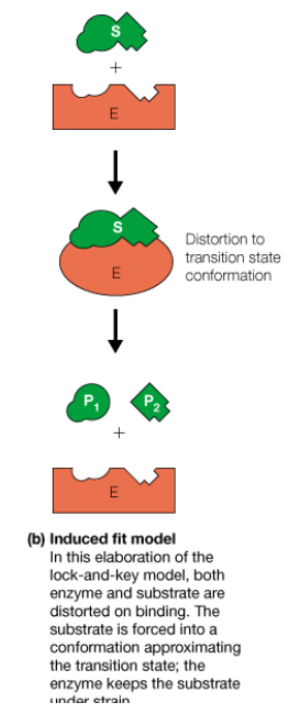
Induced fit model of enzymes
bind of substrates changes shape of enzyme
Enzymatic catalysis proceeds by one or more of what types of catalysis?
general acid/base catalysis, covalent catalysis, electrostatic stabilization, and/or proximity effects
Protein — usually plays an important role in catalysis
conformational changes
Lysozyme cleaves what?
polysaccharide
The substrate for lysozymes must do what to begin cleavage?
fit into the catalytic site
Lysozymes may be found in what food?
egg whites
As pH increases, overall charge on peptide becomes more —. As it decreases the overall charge will become more —
negative; positive
Overall charge of a protein at pi? When ph is lower than pi? When higher?
0 ; more positive; more negative
At ph>pKa, acidic side chains hold what net charge? Basic side chains?
-1;0
At ph<pKa, acidic side chains hold what net charge? Basic side chains?
0;+1
The cleavage of the saccharide bond is catalyzed by what two amino acids in the lysozyme?
aspartic acid and glutamic acid
Lysozyme catalytic mechanism (2 pathways)
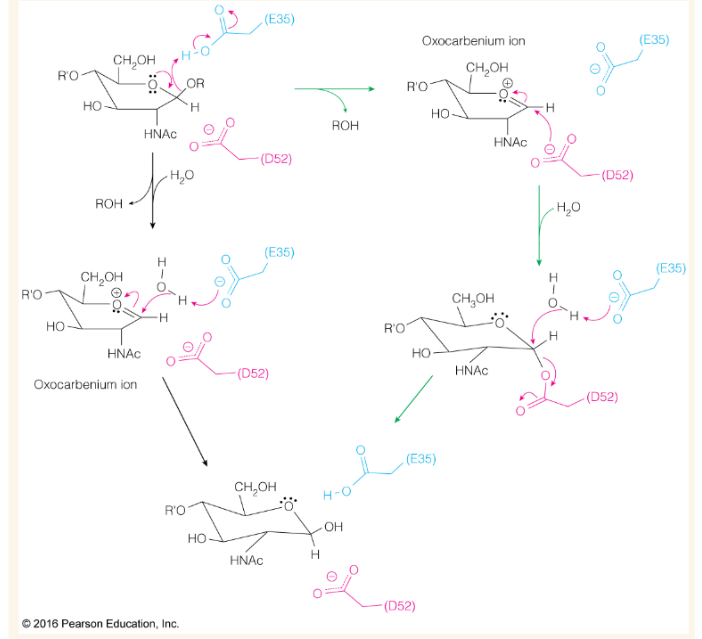
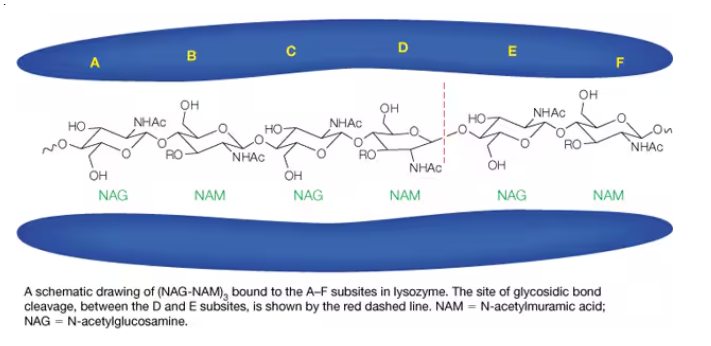
Show how and where a saccharide binds to this lysozyme. Where is the site of cleavage?
In the lysozyme mechanism E35 must be —-- in order to act as a general —-- catalysis in the rate determining step (----)
protonated; acid; oxocarbenium ion formation
In the lysozyme mechanism, E35 acts a — in the first step and a — in the last step. Why?
acid;base; it acts as an acid to donate a proton which creates the cleavage but it acts as a base in the last step in order to receive a proton from water so as to return to its original state
In the lysozyme mechanism, D52 must be —-- in order to —- the oxocarbenium ion
deprotonated to attack the oxocarbenium ion
Around what pH is lysozyme most active and why?
a pH around ~4-6. E35 must be protonated while D52 needs to be deprotonated
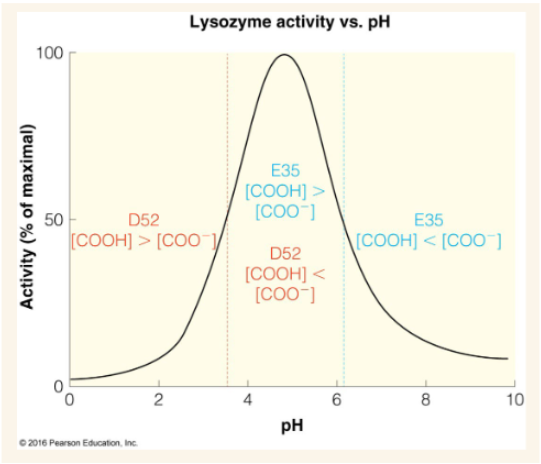
In low pH conditions why might a lysozyme be inactive?
D52 is protonated
In high pH conditions why might a lysozyme be inactive?
E35 is deprotonated
Serine Proteases
proteases that all have a critical serine nucleophile in the active site. includes trypsin, chymotrypsin and elastase
What makes the elastase pocket shallow? This shallowness causes it to be able to accommodate only —- or — residues
Val216 and Thr226 makes the pocket shallow and able to accommodate only small/medium residues
— coordinates — and — residues of substrates in the trypsin pocket
Asp189; Arg and Lys
t/f chymotrypsin’s pocket is hydrophilic
false, its hydrophobic
Chymotrypsin
serine protease with a hydrophobic pocket
3 specific amino acids found in the catalytic domain of chymotrypsin
aspartic acid, histidine and serine
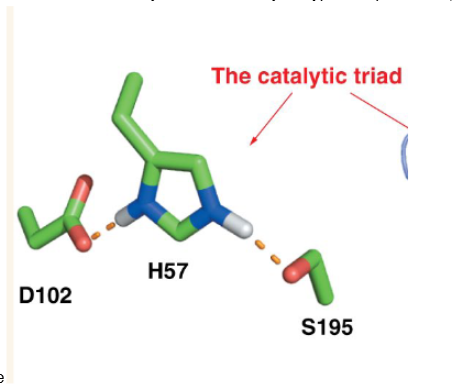
Remember, near a negative charge pKa does what?
increases
A catalytic triad consists of what? Which amino acids are these in chymotrypsin?
nucleophile(serine), general base (histidine), and an acid (aspartic acid)
Show the chymotrypsin catalytic mechanism
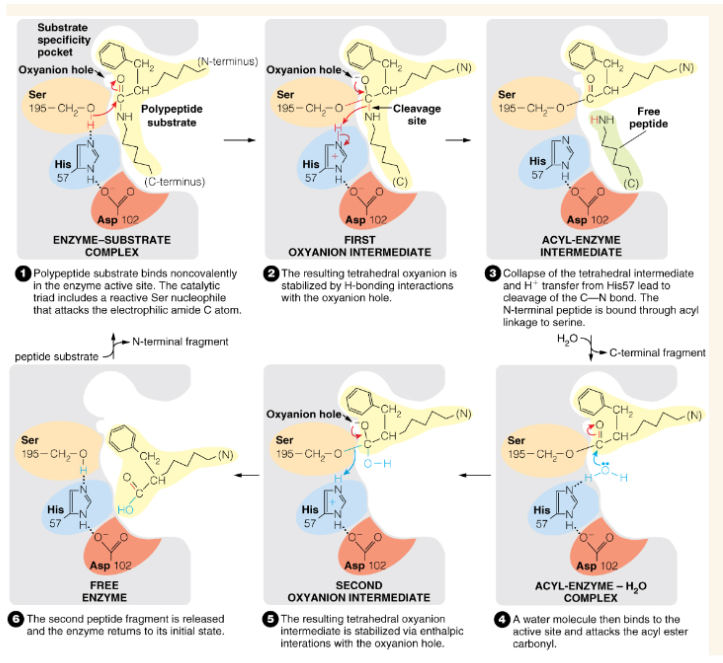
Oxyanion hole stabilizes the —- intermediate in chymotrypsin catalysis
tetrahedral
Oxyanion hole (chymotrypsin)
region of the active site of chymotrypsin which stabilizes the negatively charged tetrahedral intermediate
Cofactors
small molecules or ions that needed for some enzyme function
Coenzymes
often have complex organic structures that cannot be synthesized by some organisms. Vitamins required to make them. Slightly larger than cofactors.
Metalloenzymes
enzymes that contain one or more metal ions
A small rate constant k means what?
a slow reaction in general