Carboxylic Acids and Esters
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
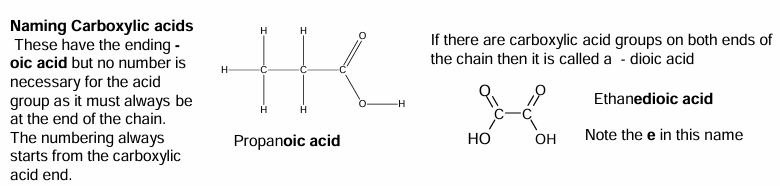
Naming carboxylic acids

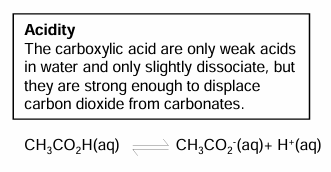
Acidity of carboxylic acids

Solubility of carboxylic acids in water

C6 actually
They also stink of vomit and body odour

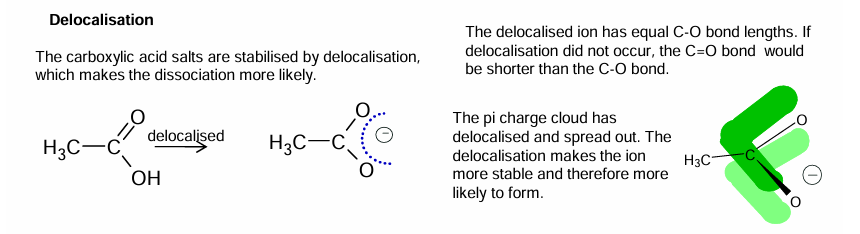
Delocalisation

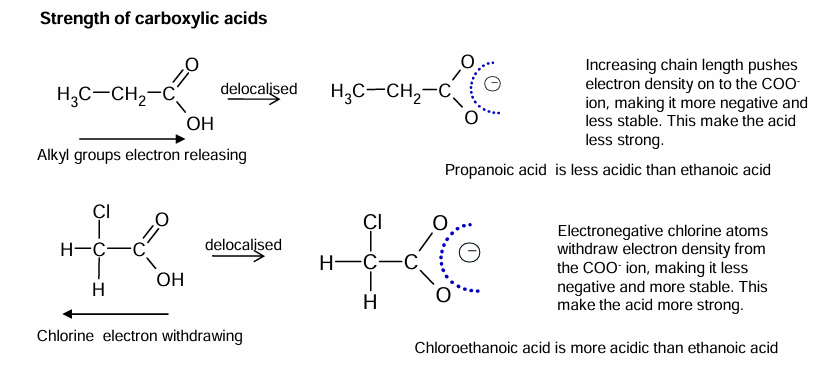
Strength of carboxylic acids

Salt formation of carboxylic acids

Put COO- Na+

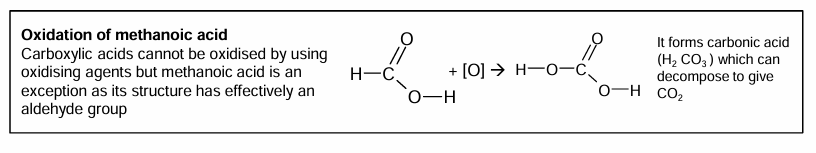
Oxidation of methanoic acid

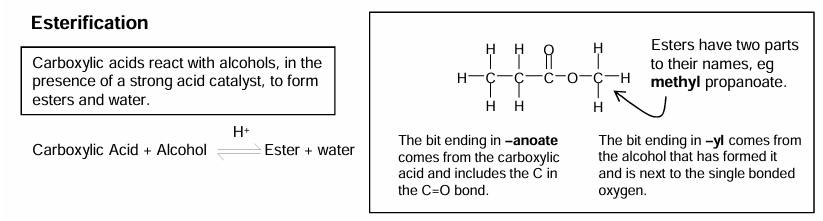
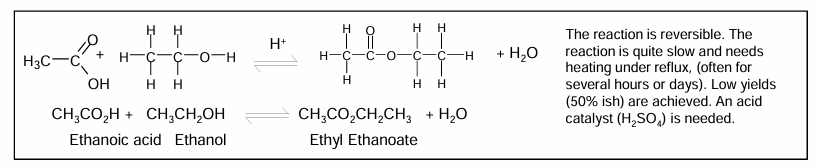
Esterification
That one oxygen will only have 2 bonds.
Concentrated acid catalyst needed
Name has 2 parts as there are 2 carbon chains

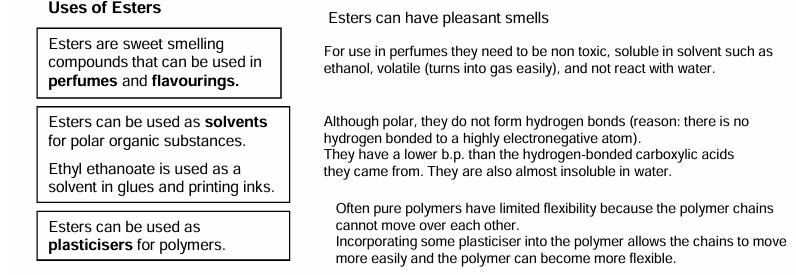
Uses of esters

Hydrolysis of esters
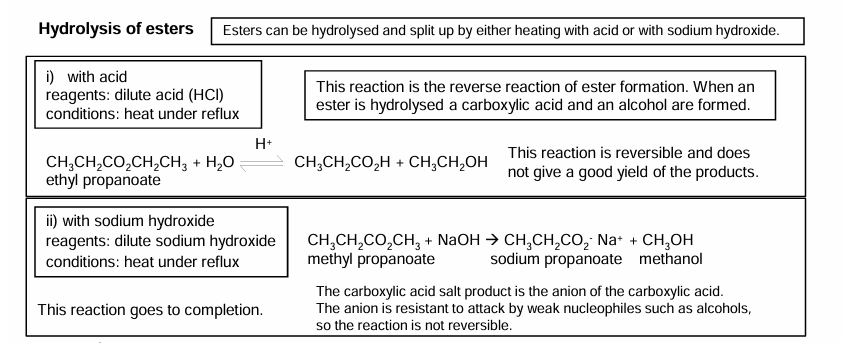

Saponification
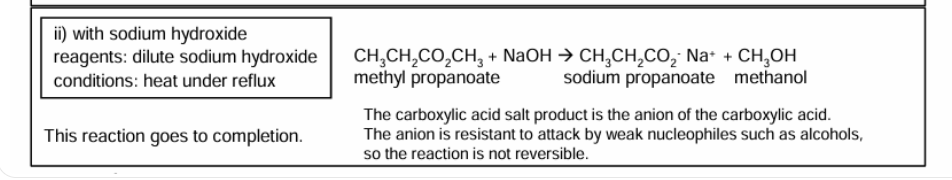
Goes to completion unlike ester hydrolysis

Fats- ignore soap for now
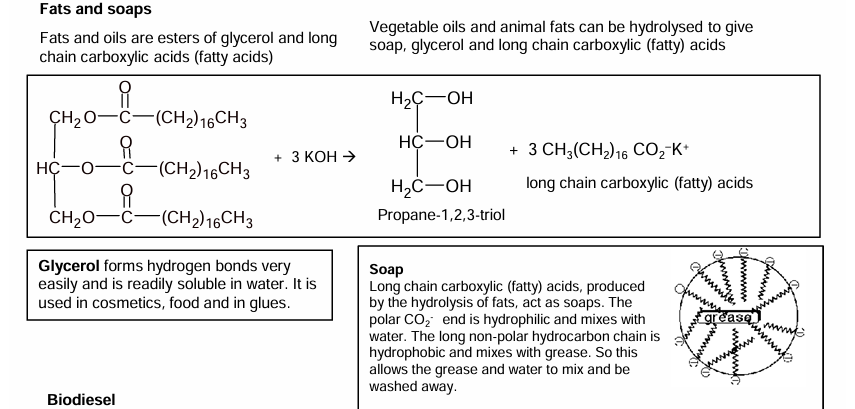
Branched= Oil
Straight= Fat
Broad group of organic molecules: fat soluble vitamins, fats, waxes, steroids
Carbon rich with little or no polar molecules
Triglycerides are composed esters. Composed of an alcohol (glycerol)- propan1,2,3-triol and three fatty acids/ carboxylic acids. Ester bond
Formed through condensation reactions.

Soap making
Saponification of lipids produces 3 salts of fatty acids and glycerol (alcohol).
Made by boiling lipids with potassium hydroxide.


Biodiesel
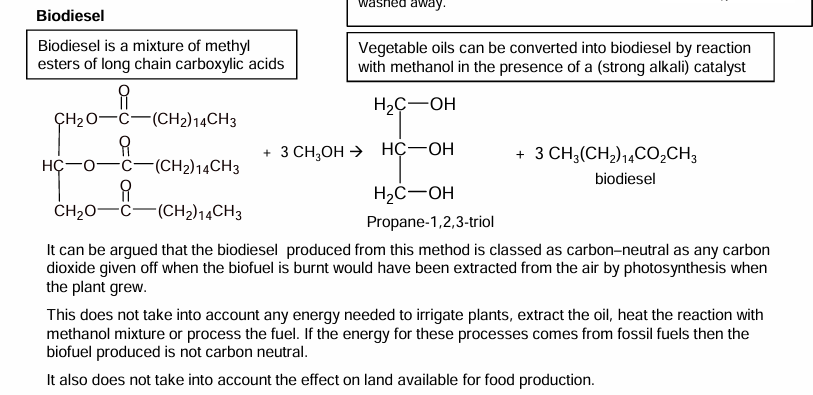
Can also be made from lipids
Triglyceride is are reacted with alcohols ( and catalysed by KOH) to form 3 methyl esters and glycerol.
3 CH3OH so one for each fatty acid
