Nucleic Acids & DNA Structure
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
New topic: Nucleic Acids & DNA Structure
Recognize the structures of the five common nucleic acid bases.
Describe the structure of nucleotides and understand the role(s) of each component.
Explain how nucleotides are used in medicine and research.
Describe (compare/contrast) the composition and structure of DNA and RNA.
(these are done individually)
the teacher said these things will be broken down to their core.
learning objectives are done individually, need to go to the textbook for clinical correlations.
Recognize the structures of the five common nucleic acid bases.
The five common bases are divided into two classes:
1. Purines (Double-Ringed Structures):
Adenine (A)
Guanine (G)
there are only TWO purines: adenine and guanine.
2.Pyrimidines (Single-Ringed Structures):
3. Cytosine (C)
4. Thymine (T)
5. Uracil (U)
Key Difference: DNA uses A, T, G, C. RNA uses A, U, G, C (Uracil replaces Thymine).
DNA uses thymine
RNA uses Uracil.
purines have a double ring structure (named because they are isolated from a compound “purine”)
pyrimidines have a single ring structure. (named because they are the modified version of purines “pyramidines” and because the pyrimidine itself is the singular six membered ring)
1. Purines (Adenine and Guanine)
Purines have a characteristic nine-membered double-ring system: a six-membered pyrimidine ring fused to a five-membered imidazole ring.
Adenine (A)
Found in: DNA and RNA
Structure:
A purine base.
Its ring system is composed of carbon and nitrogen atoms.
It has an amino group (-NH₂) attached to carbon 6 of the ring.
Pairing: In both DNA and RNA, Adenine pairs with Thymine (T) in DNA or Uracil (U) in RNA via two hydrogen bonds.
Guanine (G)
Found in: DNA and RNA
Structure:
A purine base.
It has an amino group (-NH₂) at carbon 2 and a carbonyl group (C=O) at carbon 6.
It is often represented in its keto (lactam) form, which is the predominant form in physiological conditions.
Pairing: Guanine pairs with Cytosine (C) in both DNA and RNA via three hydrogen bonds.
2. Pyrimidines (Cytosine, Thymine, and Uracil)
Pyrimidines have a simpler, pyrimidine six-membered single-ring system.
Cytosine (C)
Found in: DNA and RNA
Structure:
A pyrimidine base.
It has an amino group (-NH₂) at carbon 4 and a carbonyl group (C=O) at carbon 2.
Pairing: Cytosine pairs with Guanine (G) in both DNA and RNA.
Thymine (T)
Found in: DNA only
Structure:
A pyrimidine base.
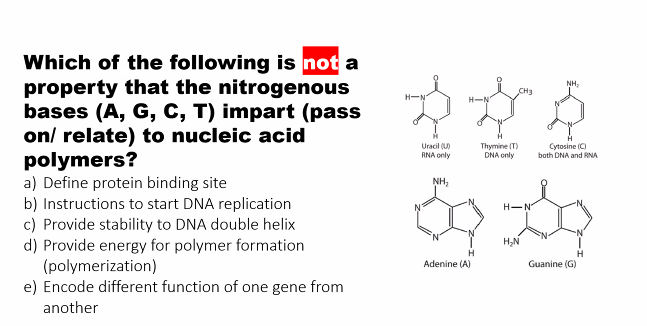
It has two carbonyl groups (C=O) at carbons 2 and 4, and a methyl group (-CH₃) at carbon 5. This methyl group is its key distinguishing feature.
Pairing: Thymine pairs with Adenine (A) in DNA.
Uracil (U)
Found in: RNA only
Structure:
A pyrimidine base.
Uracil is structurally very similar to Thymine but lacks the methyl group at carbon 5. It has two carbonyl groups (C=O) at carbons 2 and 4.
Pairing: Uracil pairs with Adenine (A) in RNA, acting as Thymine's replacement.
Summary Table for Quick Recognition
Base | Nucleic Acid | Class | Key Identifying Feature(s) |
|---|---|---|---|
Adenine (A) | DNA & RNA | Purine | Amino group (-NH₂) on the six-membered ring. |
Guanine (G) | DNA & RNA | Purine | Amino group (-NH₂) and a carbonyl (C=O) group. |
Cytosine (C) | DNA & RNA | Pyrimidine | Amino group (-NH₂) and a carbonyl (C=O) group. |
Thymine (T) | DNA only | Pyrimidine | Methyl group (-CH₃) and two carbonyl groups. |
Uracil (U) | RNA only | Pyrimidine | Looks like Thymine but without the methyl group. |
Mnemonic for Recall
Pure As Gold: Purines = Adenine and Guanine.
CUT the Py: Pyrimidines = Cytosine, Uracil, Thymine.
By recognizing the ring structure (single vs. double) and the functional groups attached (amino, carbonyl, or methyl), you can reliably identify each of the five common nucleic acid bases.
how many rings to purines have and what are the two purines?
Purines= double ring
“pure as gold”
two purines: adenine and guanine
glycosidic bond [N9-C1]
do purines have one ring?
NO!
how many rings to pyramidines have and what are the three pyramidines?
-two rings
cytosine, uracil, thymine
CUT the Py: Pyrimidines = Cytosine, Uracil, Thymine.
N-glycosidic bond: [N1-C1]
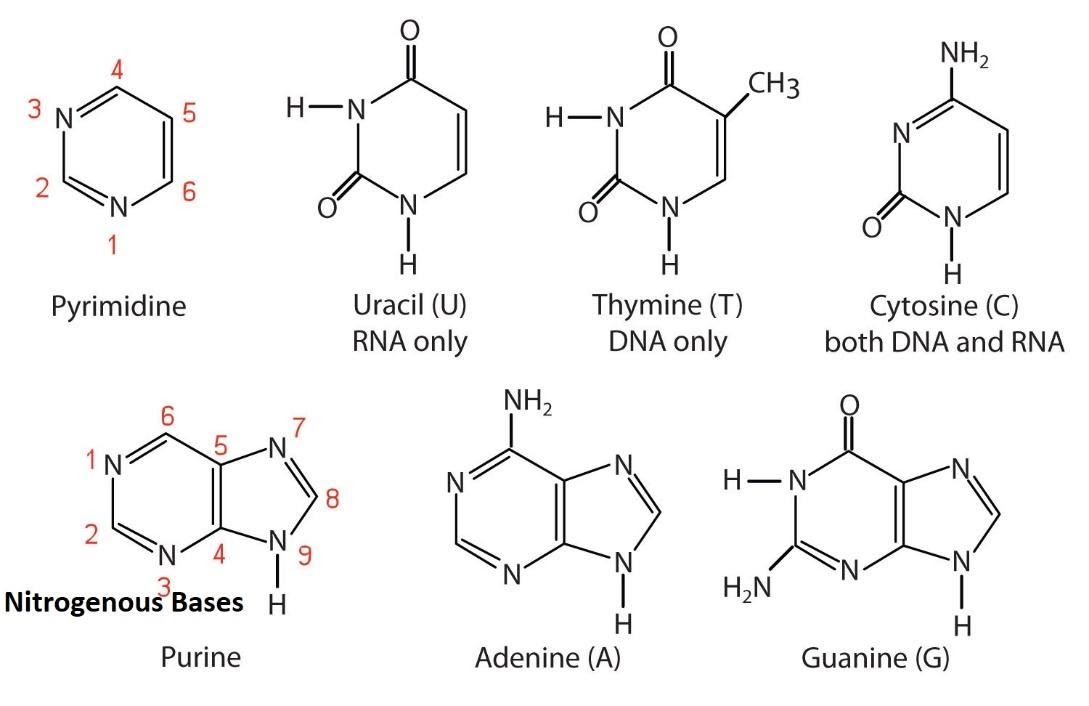
Purines: adenine
adenine is found in….
what is the structure of adenine?
what does adenine pair with? how many bonds?
Adenine (A)
Found in: DNA and RNA
Structure:
A purine base.
Its ring system is composed of carbon and nitrogen atoms.
It has an amino group (-NH₂) attached to carbon 6 of the ring.
Pairing: In both DNA and RNA, Adenine pairs with Thymine (T) in DNA or Uracil (U) in RNA via two hydrogen bonds.
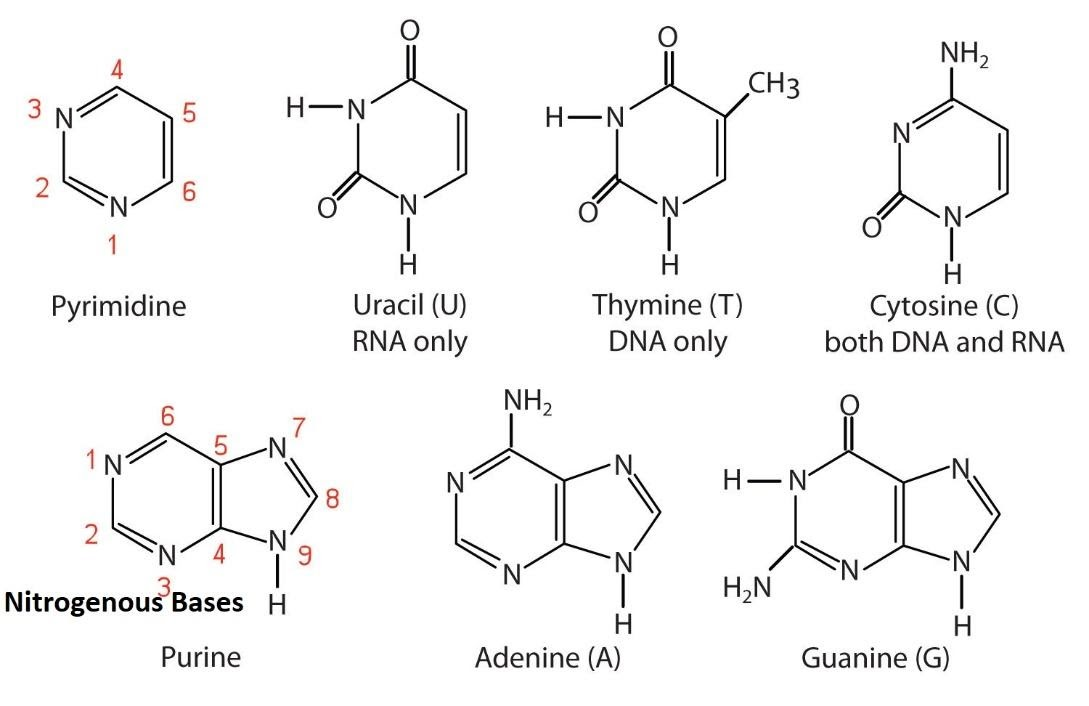
Purines: guanine
amino group position
carbonyl group position
tautomerism= which form in cells?
Guanine (G)
Found in: DNA and RNA
Structure:
A purine base.
amino group (-NH₂) at carbon 2
carbonyl group (C=O) at carbon 6.
tautomerism= often in keto (lactam) form in physiological conditions.
Guanine pairs with Cytosine (C) in both DNA and RNA via three hydrogen bonds.
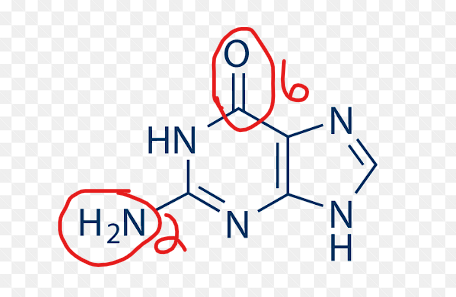
what is a distinction between adenine and guanine?
adenine DOES NOT have an oxygen.
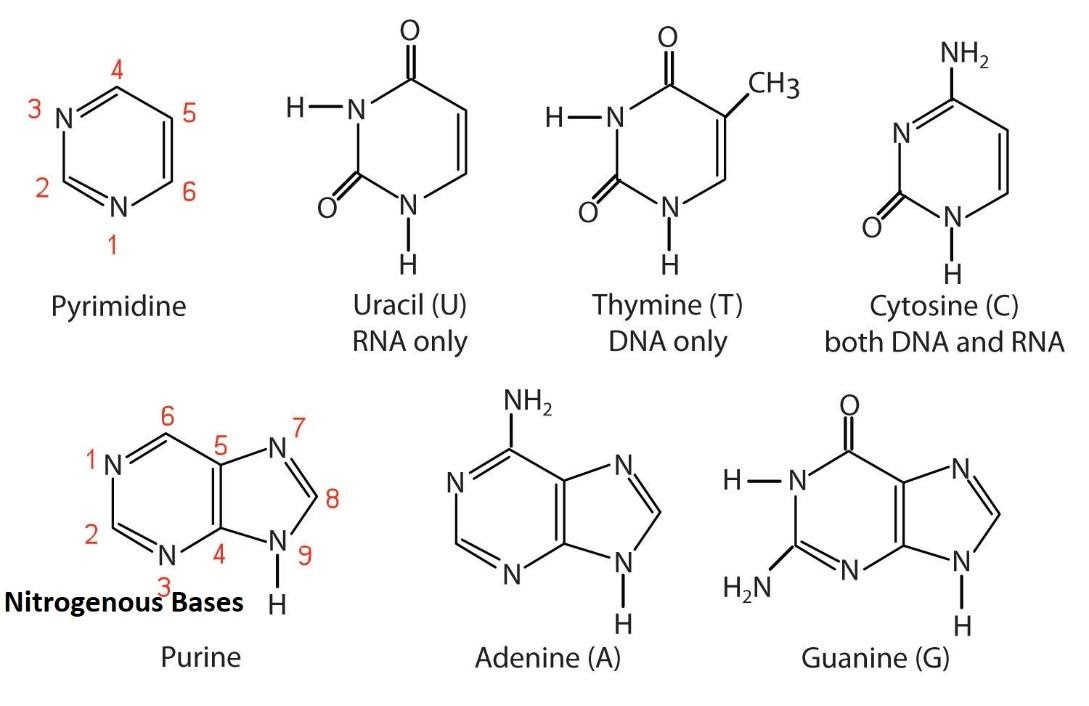
Pyramidine: cytosine
amino group on which carbon?
carbonyl group on which carbon?
Cytosine (C)
Found in: DNA and RNA
Structure:
A pyrimidine base.
It has an amino group (-NH₂) at carbon 4 and a carbonyl group (C=O) at carbon 2.
Pairing: Cytosine pairs with Guanine (G) in both DNA and RNA.
pyramidine: thymine
Thymine (T)
Found in: DNA only
Structure:
A pyrimidine base.
It has two carbonyl groups (C=O) at carbons 2 and 4, and a methyl group (-CH₃) at carbon 5. This methyl group is its key distinguishing feature.
Pairing: Thymine pairs with Adenine (A) in DNA.
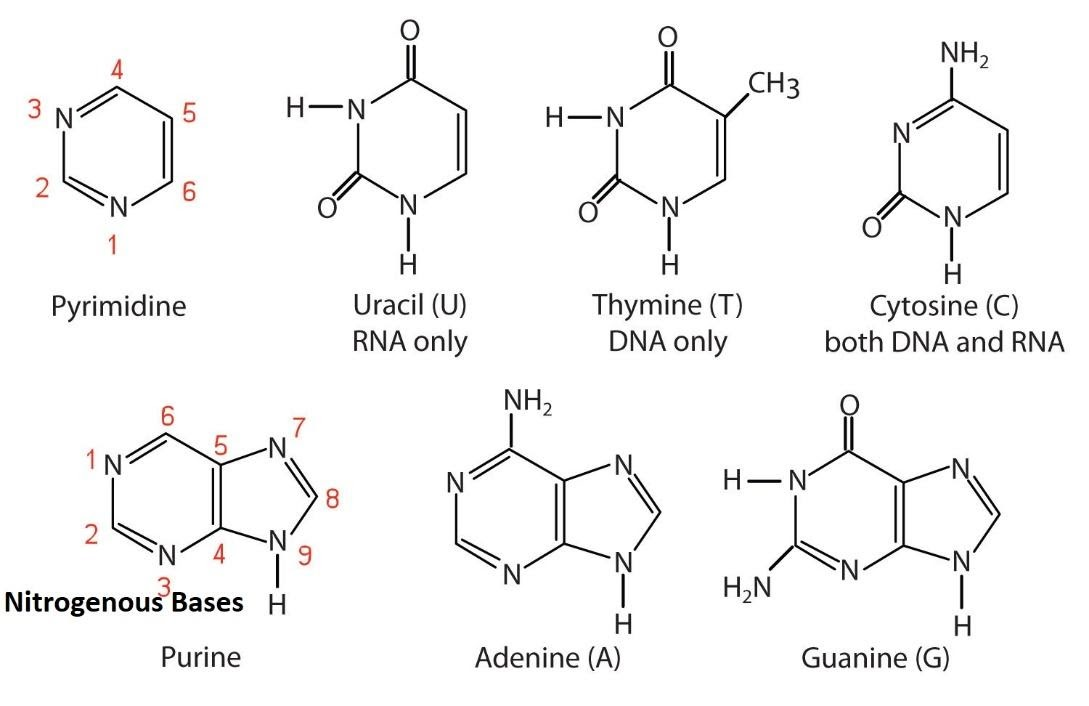
pyramidine: uracil
Uracil (U)
Found in: RNA only
Structure:
A pyrimidine base.
Uracil is structurally very similar to Thymine but lacks the methyl group at carbon 5. It has two carbonyl groups (C=O) at carbons 2 and 4.
Pairing: Uracil pairs with Adenine (A) in RNA, acting as Thymine's replacement.
what is the distinguishing feature between adenine and guanine?
oxygen
adenine DOES NOT have an OXYGEN.
guanine DOES have an OXYGEN.
what are the two distinguishing features between cytosine, uracil, and thymine?
METHYL and AMOUNT OF OXYGENS
thymine has a METHYL.
cytosine and uracil: cytosine has ONE OXYGEN, uracil has TWO OXYGENS.
why does adenosine pair with two hydrogen bonds and guanine pairs with three hydrogen bonds?
This gets to the very heart of how DNA maintains its stable, predictable structure, which is crucial for accurate replication and heredity.
The reason adenine (A) pairs with thymine (T) using two hydrogen bonds, and guanine (G) pairs with cytosine (C) using three hydrogen bonds, comes down to their precise molecular geometry and the arrangement of their hydrogen bond donors and acceptors.
Let's break it down visually and functionally.
1. The Key Players: Hydrogen Bond Donors and Acceptors
In the context of DNA base pairing:
A Hydrogen Bond Donor is a hydrogen atom covalently bonded to a strongly electronegative atom like Nitrogen (N) or Oxygen (O). This hydrogen has a partial positive charge (δ+).
A Hydrogen Bond Acceptor is a strongly electronegative atom (like Oxygen or Nitrogen) with a lone pair of electrons and a partial negative charge (δ-).
The magic of base pairing is that a donor on one base must line up perfectly with an acceptor on the other base.
2. The A-T Base Pair (2 Hydrogen Bonds)
Examine the structures of Adenine and Thymine:
Adenine: nitrogen at position 1 (acceptor) and the hydrogen on the amino group (NH₂) at position 6 (donor).
Thymine: It has a carbonyl oxygen (C=O) at position 2 (acceptor) and a hydrogen on the nitrogen at position 3 (donor).
When adenine and thymine come together, they form two perfect, complementary hydrogen bonds:
The acceptor (N1) of Adenine pairs with the donor (H-N3) of Thymine.
The donor (H-N6) of Adenine pairs with the acceptor (O=C2) of Thymine.
This creates a stable, but slightly weaker, pair.
text
Adenine (A) Thymine (T)
N1-H...O=C2
C6=NH...N3-H3. The G-C Base Pair (3 Hydrogen Bonds)
Now, examine the structures of Guanine and Cytosine:
Guanine: It has a carbonyl oxygen at position 6 (acceptor), a hydrogen on the nitrogen at position 1 (donor), and the hydrogen on the amino group (NH₂) at position 2 (donor).
Cytosine: It has a hydrogen on the nitrogen at position 3 (donor), a carbonyl oxygen at position 2 (acceptor), and a nitrogen at position 1 (acceptor) from its amino group.
When they come together, they form three perfect, complementary hydrogen bonds:
The acceptor (O=C6) of Guanine pairs with the donor (H-N4) of Cytosine (from its amino group).
The donor (H-N1) of Guanine pairs with the acceptor (N3) of Cytosine.
The donor (H-N2) of Guanine pairs with the acceptor (O=C2) of Cytosine.
This creates a stronger, more stable pair.
Guanine (G) Cytosine (C)
O=C6...H-N4
N1-H...N3
N2-H...O=C2Why Can't They Swap? The Rule of Complementarity
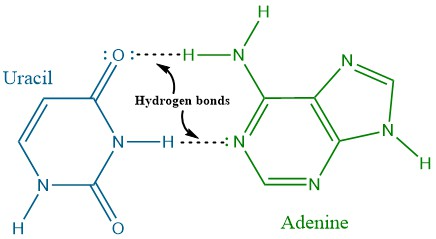
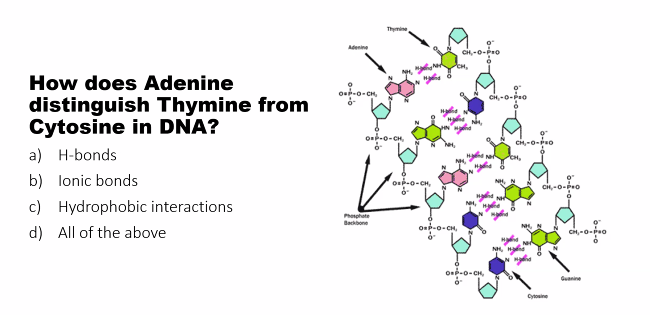
Adenine cannot pair stably with Cytosine, and Guanine cannot pair stably with Thymine. If you try to swap them, the donors and acceptors do not line up correctly (do long, wrong angles, non-polar hydrogens = extremely weak). Swapping them would make them extremely weak or non-existent. This precise "lock and key" fit is the principle of Watson-Crick complementarity (DNA double strands are mirror images of each other, A always binds to T with two hydrogen bonds, G always binds to C with three hydrogen bonds).
Consequences of 2 vs. 3 Bonds
This difference has major biological implications:
Stability: A region of DNA with a high G-C content is more thermally stable and requires a higher temperature to "melt" (separate the strands) because it takes more energy to break three hydrogen bonds per pair than two.
Evolutionary Pressure: The genetic code and mutation rates can be influenced by the strength of the base pairing. For example, an A-T pair is more prone to errors during replication than a G-C pair because it's easier to break.
DNA-Protein Recognition: The pattern of hydrogen bond donors and acceptors in the major and minor grooves of DNA (which differs between A-T and G-C pairs) allows proteins to "read" the DNA sequence without unwinding the double helix.
In summary, the number of hydrogen bonds is not an arbitrary choice but a direct and necessary consequence of the specific chemical structures of the bases, ensuring the fidelity and stability of the DNA double helix.
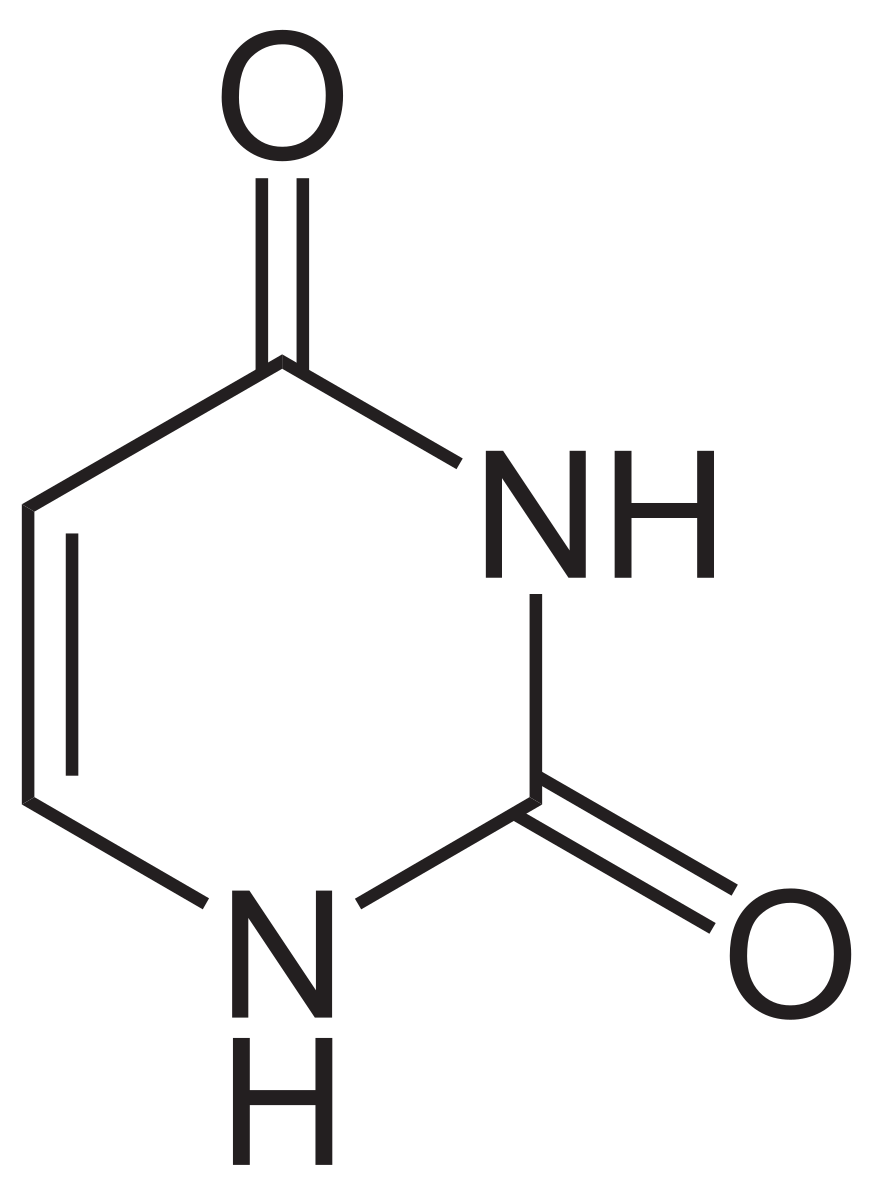
adenine and thymine hydrogen bonding
2. The A-T Base Pair (2 Hydrogen Bonds)
rule
how many hydrogen bonds are there? 2 (a-t or a-u) 3 (g-c)
look at the amount of rings in each base (to tell whether its a purine or pyramidine)
purine: adenine has NO OXYGEN, guanine HAS OXYGEN
pyramidine: cytosine has ONE OXYGEN, thymine has a METHYL, and uracil has TWO OXYGEN.
adenine and thymine: two hydrogen bonds
The N1 of adenine (acceptor) of Adenine pairs with the H-N3 (donor) of Thymine.
The donor (H-N6) of Adenine pairs with the acceptor (O=C2) of Thymine.
Character: adenine and thymine hydrogen bonding creates a stable, but slightly weaker, pair.
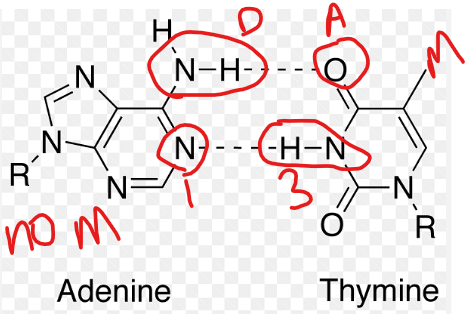
guanine and cytosine hydrogen bonding
2. The G-C Base Pair (3 Hydrogen Bonds)
rule
how many hydrogen bonds are there? 2 (a-t or a-u) 3 (g-c)
look at the amount of rings in each base (to tell whether its a purine or pyramidine)
purine: adenine has NO OXYGEN, guanine HAS OXYGEN
pyramidine: cytosine has ONE OXYGEN, thymine has a METHYL, and uracil has TWO OXYGENS.
characteristic: because there are 3 hydrogen bonds, the binding between guanine and cytosine is stronger.
why can’t hydrogen bonds between the bases be swapped?
Why Can't They Swap? The Rule of Complementarity
watson and crick rule of complementarity: the DNA double strands are mirror images of each other, A always binds to T with two hydrogen bonds and G always binds to C with three hydrogen bonds
donors and acceptors do not line up correctly (do long, wrong angles, non-polar hydrogens = extremely weak).
Swapping them would make them extremely weak or non-existent.
adenine and uracil hydrogen bonding (RNA)
rule
how many hydrogen bonds are there? 2 (a-t or a-u) 3 (g-c)
look at the amount of rings in each base (to tell whether its a purine or pyramidine)
purine: adenine has NO OXYGEN, guanine HAS OXYGEN
pyramidine: cytosine has ONE OXYGEN, thymine has a METHYL, and uracil has TWO OXYGENS.
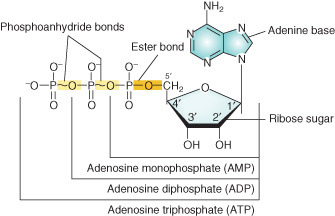
Describe the structure of nucleotides and understand the role(s) of each component.
The hierarchy of genetic material is:
Nitrogenous Base + Pentose Sugar + Phosphate Group(s) → Nucleotide → Nucleotide Chain (like DNA/RNA) → Double Helix (DNA)
The Three Components of a Nucleotide
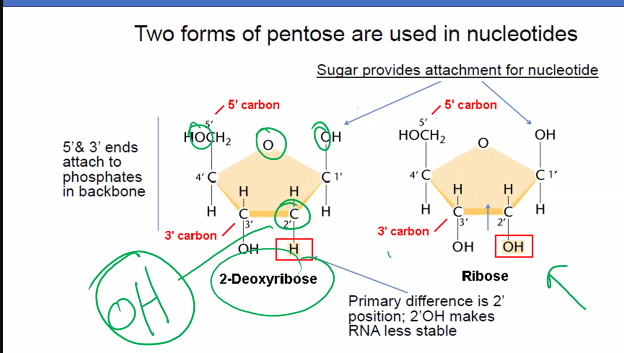
A nucleotide is the basic building block of nucleic acids, like DNA and RNA, and is made of three parts covalently bonded together: a five-carbon sugar, a phosphate group, and a nitrogenous base.
1. Nitrogenous Base “information carrying” part of the molecule.
What it is: A carbon-nitrogen ring structure that acts as the "information-carrying" part of the molecule.
Structure & Types: As discussed in the previous section, there are five common bases:
Purines (double ring): Adenine (A) and Guanine (G).
Pyrimidines (single ring): Cytosine (C), Thymine (T - DNA only), Uracil (U - RNA only).
Role(s):
Information Storage: The specific sequence of these bases (A, T, C, G in DNA) encodes genetic information, much like letters in an alphabet form words and sentences.
Molecular Recognition: Bases form specific hydrogen bonds with their complementary partners (A with T/U, G with C). hydrogen bonding is the foundation of DNA replication and RNA transcription.
Cellular Signaling: Some nucleotides, like cyclic AMP (cAMP), use their base as part of a key signaling molecule that regulates cellular processes.
KNOWLEDGE GAP: CELLULAR SIGNALING.
2. Pentose Sugar “backbone”
What it is: A 5-carbon sugar molecule that forms the structural "backbone" of the nucleic acid chain.
Structure & Types:
The key difference between DNA and RNA lies in this sugar.
In DNA: The sugar is Deoxyribose. It has a hydrogen (H) atom attached to the 2' carbon (hence "deoxy-").
In RNA: The sugar is Ribose. It has a hydroxyl (OH) group attached to the 2' carbon.
Role(s):
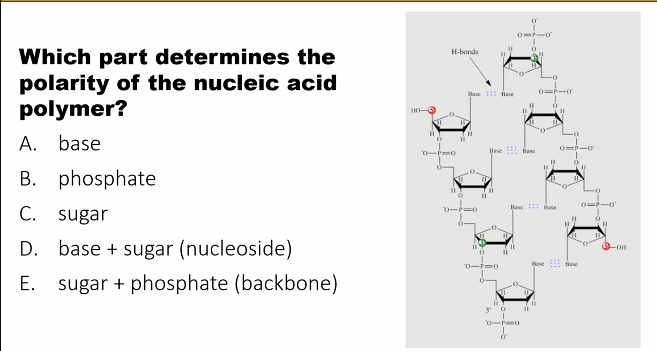
Structural Scaffold: The sugars link together via phosphate groups to form a strong, stable backbone for the long nucleic acid polymer.
Identity Determinant: The presence of deoxyribose defines a molecule as DNA, and ribose defines it as RNA.
The extra oxygen in ribose makes RNA more chemically reactive and less stable than DNA. (KNOWLEDGE GAP)
Directionality: The carbon atoms in the sugar (numbered 1' to 5') define the direction of the nucleic acid chain. The 5' carbon of one sugar connects to the 3' carbon of the next, giving the strand a 5' to 3' directionality, which is critical for enzymes that read and copy DNA/RNA.
3. Phosphate Group “connecting the 5' carbon of one sugar to the 3' carbon of the next”
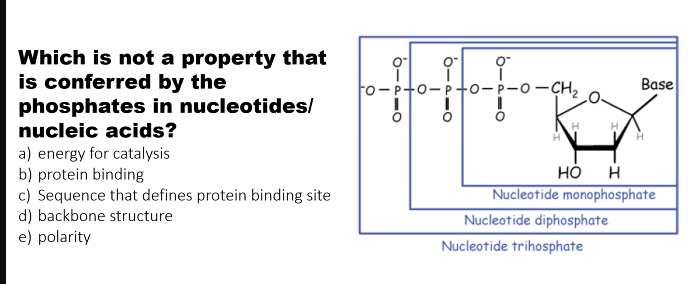
What it is: A functional group consisting of a phosphorus atom bonded to four oxygen atoms.
Structure: A single nucleotide can have one, two, or three phosphate groups (e.g., ATP has three). In a nucleic acid polymer, the phosphate group is part of the linkage between sugars.
Role(s):
Polymer Formation: the phosphate group forms the "bridge" in the phosphodiester bond, connecting the 5' carbon of one sugar to the 3' carbon of the next. This creates the sugar-phosphate backbone.
Energy Currency: Nucleotides with multiple phosphates (like ATP - Adenosine Triphosphate) are the primary energy carriers in the cell. The bonds between these phosphates are high-energy, and breaking them releases energy to drive chemical reactions.
Negative Charge: The phosphate groups are negatively charged at cellular pH. This gives the entire DNA/RNA molecule a negative charge, which is important for its interaction with proteins (like histones) and for its overall structure.
How the Components Are Assembled
Nucleoside = Base + Sugar
The nitrogenous base is attached to the 1' carbon of the sugar via a glycosidic bond.
Examples: Adenosine, Guanosine, Cytidine, Thymidine, Uridine.
Nucleotide = Nucleoside + Phosphate Group(s)
The phosphate group is attached to the 5' carbon of the sugar.
Examples: Adenosine Monophosphate (AMP), Deoxyguanosine Triphosphate (dGTP).
Analogy: A Nucleotide as a Train Car
Imagine a nucleotide is a single car on a freight train (the DNA/RNA strand).
The Nitrogenous Base is the cargo inside the car. It's the unique part that specifies what is being carried (an A, T, G, or C).
The Pentose Sugar is the body and frame of the car. It gives the structure its shape and connects it to the other cars. The difference between a DNA car and an RNA car is the type of frame used.
The Phosphate Group is the metal coupler that links one car to the next, forming the long train.
Summary of Roles in a DNA/RNA Polymer
Component | Primary Role in a Nucleic Acid Polymer |
|---|---|
Nitrogenous Base | Information. Forms the genetic code through its sequence. |
Pentose Sugar | Structure & Identity. Forms the backbone and distinguishes DNA from RNA. |
Phosphate Group | Linkage & Charge. Connects nucleotides and gives the molecule a negative charge. |
By combining these three components, nucleotides become the versatile monomers capable of storing genetic information, translating it into proteins, and serving as key energy and signaling molecules for all life.
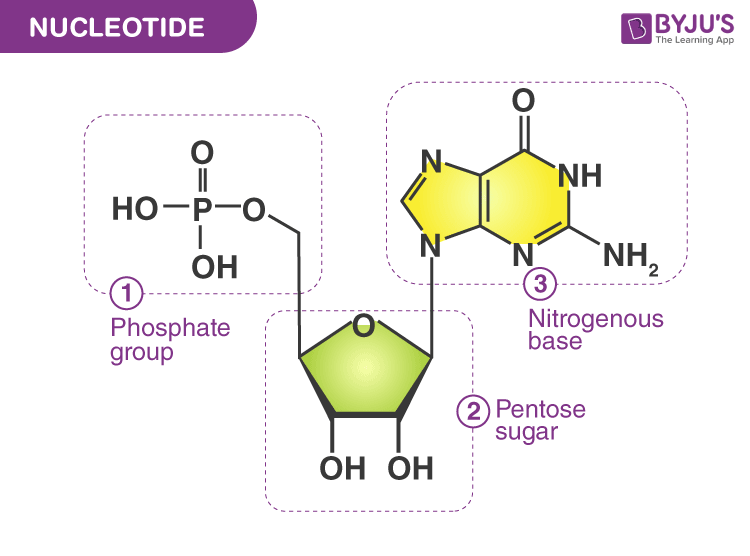
Describe the structure of nucleotides and understand the role(s) of each component (structure of a nucleotide)
nitrogenous base (A,T,U,C,G): carries information
pentose sugar: forms backbone
phosphate: attaches sugar to sugar, charge and energy
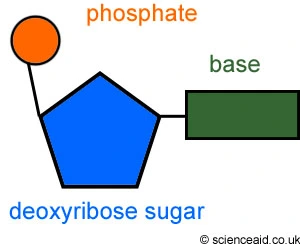
what is an alpha, beta, gamma phosphate group?
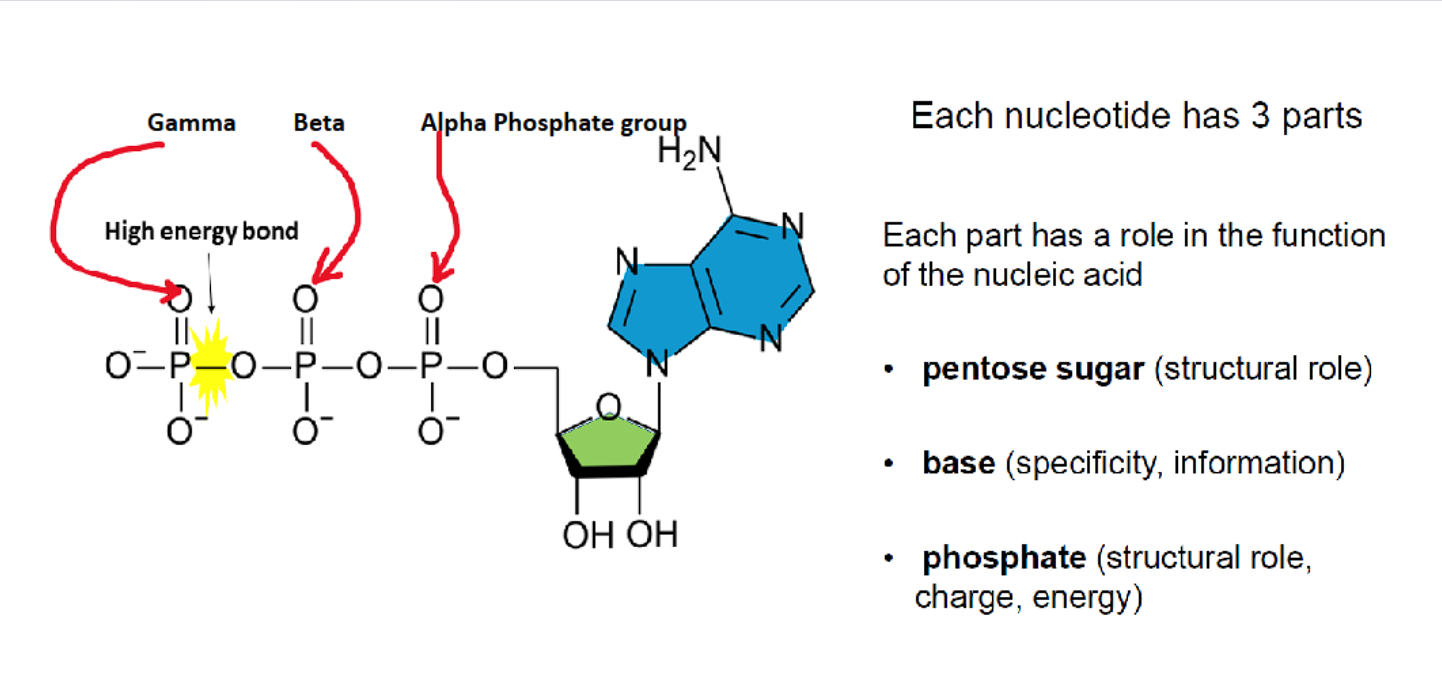
HOW is the 5’ carbon CONNECTED to the 3’ carbon of the next molecule?
connecting the 5' carbon of one sugar to the 3' carbon of the next.)
why is ATP the energy molecule?
when the bonds of ATP are broken, there is a HUGE amount of energy being released.
which bond on ATP when hydrolyzed releases energy?
the massive energy release comes specifically from the hydrolysis of the phosphoanhydride bonds, not the phosphoester bond.
why is a negative charge on DNA and RNA useful?
negative charge (the phosphates coat DNA and RNA with a negative charge (a negative charge at cellular pH is useful for interactions with proteins, such as chaperones).
what is the difference between nucleoside and nucleotide?
answer: phosphate groups
nucleoside: Nucleoside = nitrogenous Base + pentose Sugar
The nitrogenous base is attached to the 1' carbon of the sugar via a glycosidic bond.
Examples: Adenosine, Guanosine, Cytidine, Thymidine, Uridine.
nucleotide: nitrogenous base + pentose sugar + Phosphate Group(s)
The phosphate group is attached to the 5' carbon of the sugar.
Examples: Adenosine Monophosphate (AMP), Deoxyguanosine Triphosphate (dGTP).
DNA and RNA are made of NUCLEOTIDES!
what is the difference between an nucleoside monophosphate, diphosphate, and triphosphate?
where is the glycosidic bond located?
monophosphate: one phosphate
diphosphate: two phosphates
triphosphates: three phosphates
glycosidic bond is located between the base and sugar.
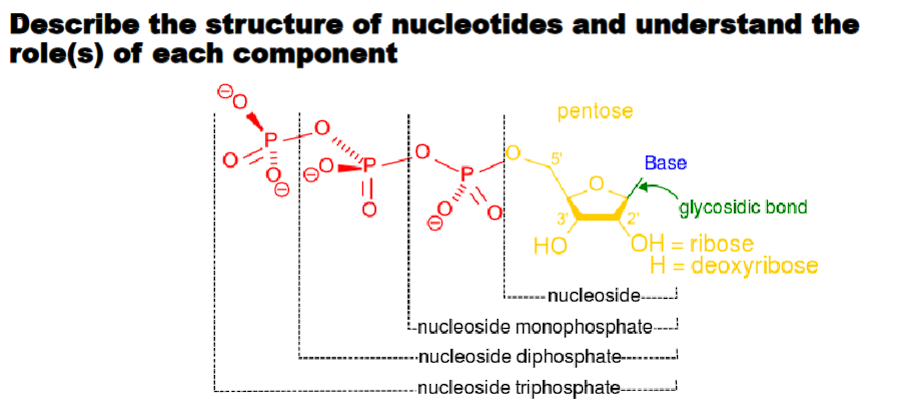
2-deoxyribose: means that carbon 2 does not have an oxygen.
2-deoxyribose: DNA (D: deoxyribose)
deoxy means without oxygen.
Ribose: RNA (R: ribose)
2’OH makes RNA more reactive in alkali conditions (ribozyme)
Explain how nucleotides are used in medicine and research.
1. Medicine: Nucleotides as Therapeutics and Diagnostics
In medicine, the primary use of nucleotides is to interfere with pathological processes, often in cancer and viral infections.
A. Antiviral and Anticancer Drugs (Nucleoside/Nucleotide Analogs)
analog: similar structure
This is the most prominent medical application. These drugs are synthetic molecules that mimic the structure of natural nucleosides/tides but are subtly modified.
Mechanism of Action:
False Substitution: They are taken up by cells and converted into the active triphosphate form.
Chain Termination: When a virus or cancer cell replicates its DNA/RNA, it mistakenly incorporates the drug molecule into the growing chain.
Termination: The analog lacks the proper chemical group for the next nucleotide to attach, causing premature termination of the DNA/RNA chain. This halts the replication of the virus or the proliferation of the cancer cell.
Key Examples:
Antivirals:
Acyclovir (Zovirax): Used for herpes viruses. It is a guanosine analog.
Azidothymidine (AZT): The first drug approved for HIV. It is a thymidine analog.
Remdesivir: Used for COVID-19 and other RNA viruses. It is an adenosine analog that evades the virus's proofreading mechanism.
Anticancer Drugs:
Cytarabine: Used to treat leukemias. It is a cytosine analog.
5-Fluorouracil (5-FU): A widely used drug for solid tumors (e.g., colon, breast). It is a uracil analog that inhibits a key enzyme (thymidylate synthase) involved in thymidine production, starving the cancer cell of a critical DNA building block.
Gemcitabine: Used for pancreatic, lung, and bladder cancers. It is a cytidine analog.
B. Molecular Diagnostics
Nucleotides are essential for detecting and identifying pathogens and genetic mutations.
PCR (Polymerase Chain Reaction): amplifies millions of copies of a DNA sequence
DNA Sequencing: Modern sequencing technologies (Next-Generation Sequencing) rely on fluorescently labeled nucleotides. As each nucleotide is incorporated into a DNA strand, a detector reads its fluorescent color, determining the sequence (A, T, C, G). This is crucial for identifying cancer mutations, diagnosing genetic disorders, and tracking pathogen outbreaks.
C. mRNA Vaccines
This is a revolutionary application that uses a nucleotide-based molecule to instruct our own cells to make a viral protein.
The Nucleotides Component: The "active ingredient" is Messenger RNA (mRNA), which is a long chain of nucleotides.
Mechanism: The mRNA is engineered to carry the genetic code for a specific viral protein (e.g., the SARS-CoV-2 spike protein). Once injected, our cells use their own machinery to read this mRNA and produce the viral protein. Our immune system then recognizes this foreign protein and mounts a protective response. The nucleotides in the mRNA are the literal carriers of the genetic instructions.
D. Signaling and Physiology
Cyclic AMP (cAMP): A nucleotide (derived from ATP) that acts as a vital second messenger inside cells. It relays signals from hormones (like adrenaline) to trigger various cellular responses. Some drugs work by modulating cAMP levels.
2. Research: Nucleotides as Fundamental Tools
In the lab, nucleotides are the workhorses for manipulating and studying genes.
A. DNA Sequencing and Synthesis
As mentioned above, nucleotides are the core component of all DNA sequencing technologies, enabling the Human Genome Project and personalized genomics.
Gene Synthesis: Scientists can now "print" DNA by chemically linking nucleotides in any desired order to create synthetic genes for research, or even for producing synthetic biology products (e.g., insulin).
B. PCR (Polymerase Chain Reaction)
Beyond diagnostics, PCR is a foundational research tool used for:
Gene Cloning: Amplifying a specific gene to insert it into another organism.
Gene Expression Analysis: Measuring how active a gene is.
Forensics: Amplifying tiny amounts of DNA from a crime scene.
Ancient DNA Studies: Amplifying degraded DNA from fossils.
C. Cell Biology and Biochemistry
ATP (Adenosine Triphosphate): The universal energy currency. Researchers study ATP to understand cellular metabolism, and they use purified ATP to power energy-dependent reactions in test tubes.
Labeling and Detection:
Radioactive Labels: Nucleotides tagged with radioactive phosphorus (³²P) are used to create DNA probes that can find and bind to complementary sequences, allowing scientists to visualize specific genes.
Fluorescent Labels: Nucleotides tagged with fluorescent dyes are used in techniques like DNA microarrays and fluorescent in situ hybridization (FISH) to visualize the location of genes on chromosomes or within cells.
D. Structural Biology
X-ray Crystallography & Cryo-EM: To understand how proteins interact with DNA/RNA, researchers often co-crystallize them. The nucleotide sequence used can determine whether a crystal forms and how well it diffracts, enabling the determination of 3D structures at atomic resolution.
Summary Table
Field | Application | How Nucleotides are Used |
|---|---|---|
Medicine | Antiviral/Anticancer Drugs | Chain Termination. Analogs are incorporated into DNA/RNA during replication, halting the process. |
mRNA Vaccines | Genetic Instruction. mRNA nucleotides provide the code for our cells to produce a specific antigen. | |
Molecular Diagnostics (PCR) | Amplification. Free nucleotides (dNTPs) are used by DNA polymerase to copy and amplify target DNA. | |
Research | DNA Sequencing | Template & Substrate. The DNA to be sequenced is the template; fluorescently labeled nucleotides are the substrates for synthesis. |
Gene Cloning & Analysis (PCR) | Targeted Amplification. Nucleotides are used to make millions of copies of a specific gene for study. | |
Cell Signaling & Metabolism | Energy & Signaling. ATP is studied and used as an energy source; cAMP is studied as a signaling molecule. | |
Gene Detection | Labeled Probes. Radioactive or fluorescent nucleotides are used to tag DNA probes to find specific sequences. |
In summary, from stopping a virus in its tracks to reading the entire human genetic code, the applications of nucleotides are a powerful testament to how understanding fundamental biology can drive revolutionary advances in both medicine and science.
how are nucleotides used in medicine?
Antiviral and Anticancer Drugs (Nucleoside/Nucleotide Analogs)
molecule diagnostic (PCR and DNA sequencing)
mRNA Vaccines
Signaling and Physiology (cAMP): second messenger
1. Medicine: Nucleotides as Therapeutics and Diagnostics
Antiviral and Anticancer Drugs (Nucleoside/Nucleotide Analogs)
Molecular Diagnostics DNA
PCR (Polymerase Chain Reaction): amplifies millions of copies of a DNA sequence
DNA sequencing: Determines the exact order of nucleotides (A, T, C, G) in a piece of DNA to detect mutations and genetic disorders
mRNA Vaccines
instructs our own cells to make a viral protein so we can become immune to it.
Signaling and Physiology (cAMP)
Research: Nucleotides as Fundamental Tools
A. DNA Sequencing and Synthesis
B. PCR (Polymerase Chain Reaction)
C. Cell Biology and Biochemistry
D. Structural Biology
In the lab, nucleotides are the workhorses for manipulating and studying genes.
DNA Sequencing and Synthesis
PCR (Polymerase Chain Reaction)
Cell Biology and Biochemistry
Structural Biology
Describe (compare/contrast) the composition and structure of DNA and RNA.
DNA is the stable, long-term genetic database of the cell, using deoxyribose sugar and the base Thymine. It exists as a double-stranded helix.
RNA is the versatile, short-term messenger and functional molecule, using ribose sugar and the base Uracil. It is primarily single-stranded and can fold into diverse structures.
Detailed Comparison
Feature | DNA (Deoxyribonucleic Acid) | RNA (Ribonucleic Acid) |
|---|---|---|
1. Sugar-Phosphate Backbone | Deoxyribose (lacks an oxygen atom on the 2' carbon). This makes the molecule more chemically stable. | Ribose (has a hydroxyl, -OH, group on the 2' carbon). This makes RNA more reactive and less stable. |
2. Nitrogenous Bases | Purines: Adenine (A), Guanine (G) | Purines: Adenine (A), Guanine (G) |
3. Base Pairing | A pairs with T (2 hydrogen bonds) | A pairs with U (2 hydrogen bonds) |
4. Overall Structure | Double-stranded helix (typically a right-handed B-form). The two strands are antiparallel (run in opposite directions). | Primarily single-stranded, but can fold into complex 3D shapes (e.g., hairpin loops, stem-loops) due to intra-strand base pairing. |
5. Stability | Highly stable. The deoxyribose sugar is resistant to hydrolysis, and the double-stranded structure protects the bases in the hydrophobic core. | Less stable. The ribose sugar is prone to hydrolysis, especially in alkaline conditions, due to the 2' OH group. Its single-stranded nature is more vulnerable to enzymes. |
6. Length | Very long polymer (millions of base pairs in a single chromosome). | Relatively short polymer (a few dozen to a few thousand nucleotides). |
7. Key Types | One primary type (genomic DNA), with variations in packaging. | Multiple types with specialized functions: |
Visual and Descriptive Breakdown
1. The Sugar: The Core Chemical Difference
DNA: The sugar is deoxyribose. The "deoxy-" means it is missing an oxygen atom on the 2' carbon of the sugar ring. It has just a hydrogen atom (-H). This small difference has huge consequences for stability.
RNA: The sugar is ribose. It has a hydroxyl group (-OH) on the 2' carbon. This group makes RNA more chemically reactive and susceptible to breakdown.
2. The Bases: Thymine vs. Uracil
DNA uses Thymine (T). Thymine has a methyl group (-CH₃) that Uracil lacks.
RNA uses Uracil (U). Uracil is functionally similar to Thymine in base-pairing (it also pairs with Adenine), but it lacks the methyl group.
The use of Thymine in DNA is an evolutionary advantage. Cytosine can spontaneously deaminate to form Uracil. If DNA used Uracil naturally, the cell's repair mechanisms wouldn't be able to tell a damaged "C" (which became U) from a legitimate "U". Because DNA uses T, any U found in DNA is instantly recognized as a mistake and repaired.
3. The Structure: Double Helix vs. Functional Folds
DNA's Double Helix: The famous Watson-Crick model. Two polynucleotide strands twist around each other like a spiral staircase.
The sugar-phosphate backbones form the "rails" on the outside.
The paired bases form the "steps" on the inside, held together by hydrogen bonds.
The two strands are antiparallel: one runs 5'→3' and the other runs 3'→5'.
Function: This structure is perfect for stable information storage. It protects the genetic code (the bases) inside the helix and allows for accurate replication.
RNA's Variable Structures: RNA is typically a single strand, but it can fold back on itself.
Where complementary sequences exist within the same strand, base pairing occurs (A-U, G-C), forming regions of double helix.
This creates complex 2D and 3D shapes like hairpins, stem-loops, and bulges.
Function: This structural diversity allows RNA to perform many active roles in the cell. For example:
tRNA folds into a cloverleaf shape to carry amino acids.
rRNA folds into a complex 3D structure that forms the catalytic core of the ribosome.
what is the difference between dna and rna in terms of sugar and base?
DNA is the stable, long-term genetic database of the cell, using deoxyribose sugar and the base Thymine. It exists as a double-stranded helix.
RNA is the versatile, short-term messenger and functional molecule, using ribose sugar and the base Uracil. It is primarily single-stranded and can fold into diverse structures.
DNA has DNA, there are different types of RNA (tRNA, mRNA, etc)
tRNA transfers amino acids into ribosomes
rRNA: folds into a complex 3D structure that forms the catalytic core of the ribosome.
DNA has antiparallel strands: meaning that one strand has a 5' → 3' orientation, while the other strand has the opposite, 3' → 5' orientation.
Nucleic acid strands are always synthesized and "read" in the 5' to 3' direction. So, we don't say a strand "runs" 3'->5' in a functional sense. We define its orientation based on the 5' to 3' direction of its sugar-phosphate backbone.
DNA is the blueprint, RNA is the working copy.
what direction are nucleotide strands made in?
Nucleic acid strands are always synthesized and "read" in the 5' to 3' direction.
what is the difference of DNA and RNA in terms of type?
DNA has DNA, there are different types of RNA (tRNA, mRNA, etc)
tRNA transfers amino acids into ribosomes
rRNA: folds into a complex 3D structure that forms the catalytic core of the ribosome.
what is the sugar in RNA?
D-ribose
what is the sugar in DNA?
2-deoxy-D-ribose
in what position is the base attached to and what type of bond is between the sugar and base?
1st position
glycosidic bond between the sugar and base.
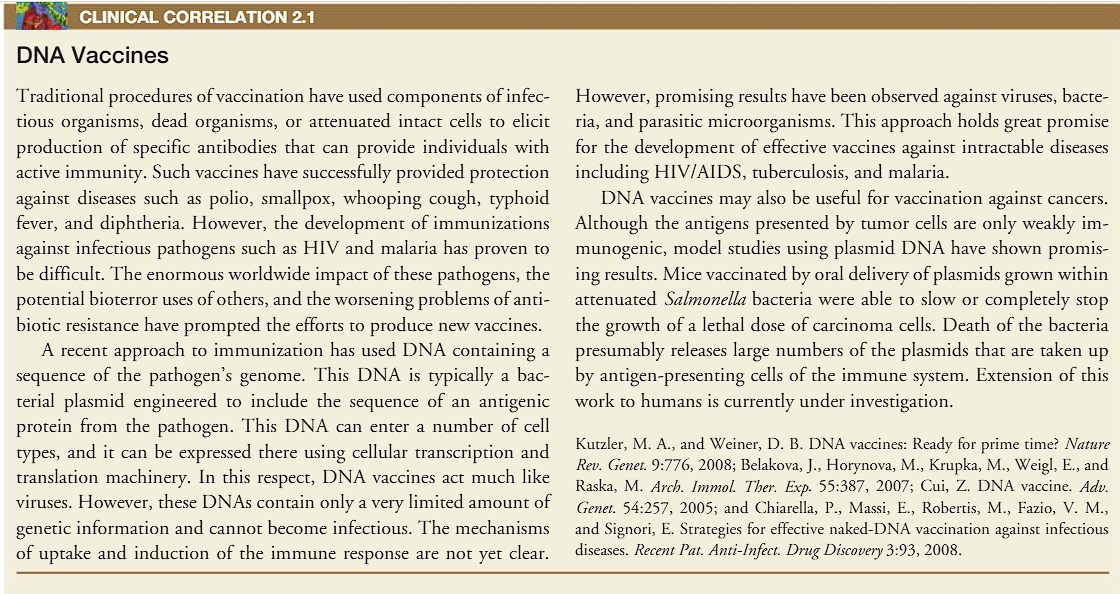
DNA Vaccines
b)

A)
C)
e)
C)\


d)
D)
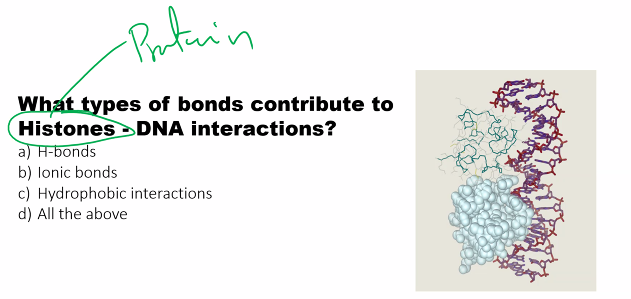
a)
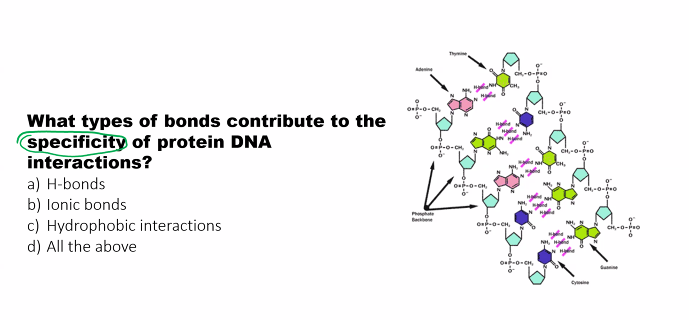
d)

a)
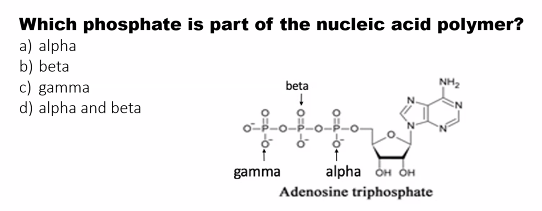
a)
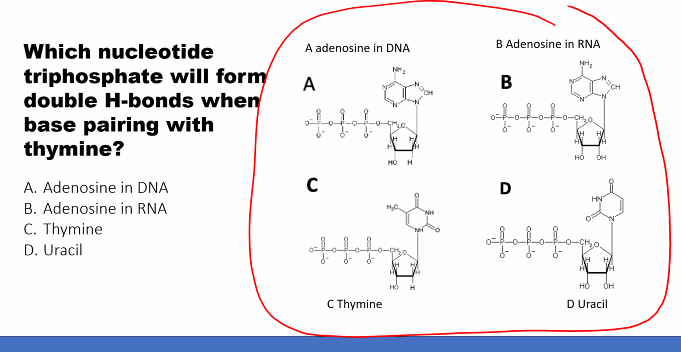
d)

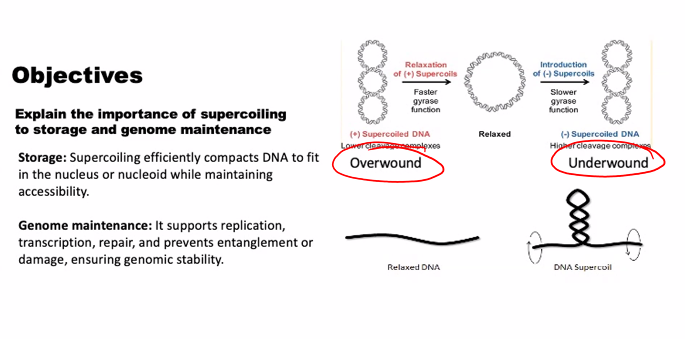
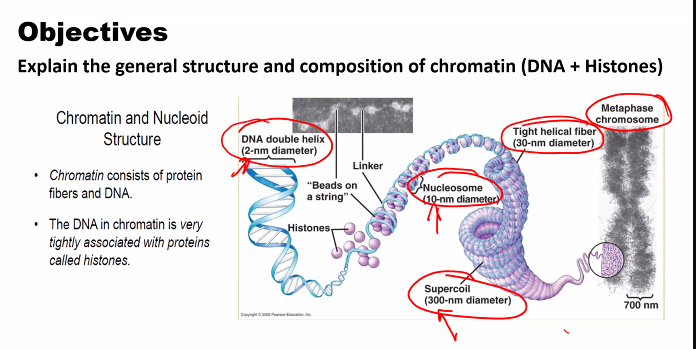
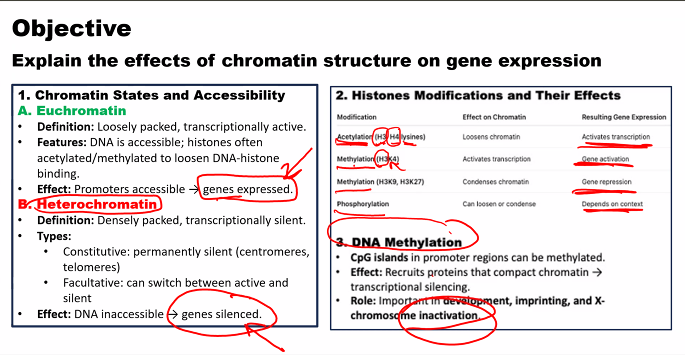
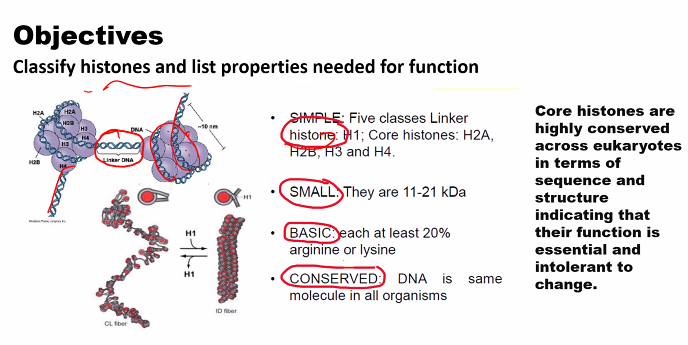
e)

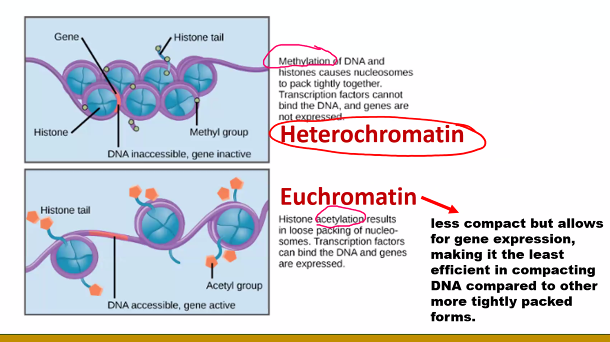
look at the picture
questions can also come from demethylation and deacetylation.
E)
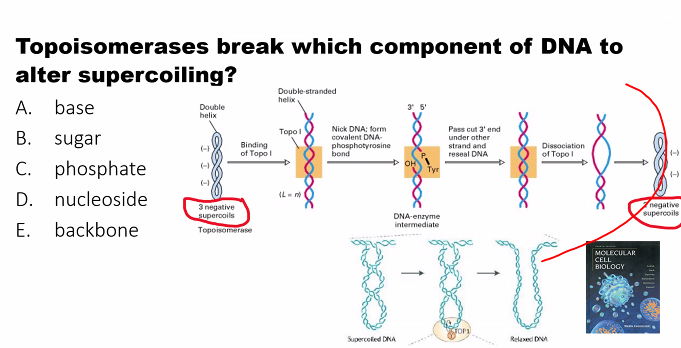
E)
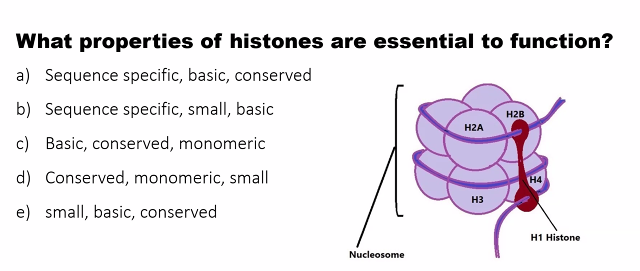
D)

c)
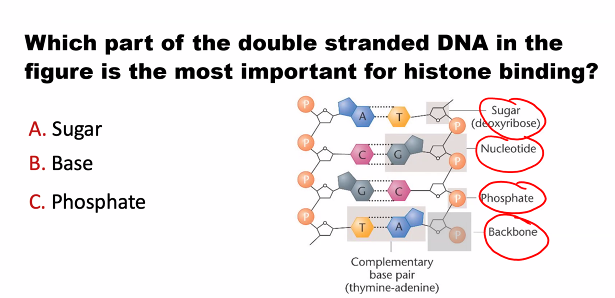
E)
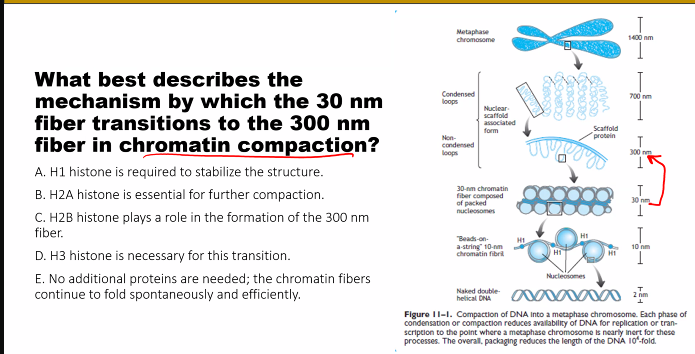
d)

C)
