Thermodynamics Final Formulas
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Activity Coefficient
gamma is activity coef
a is activity
X is molar fraction. A/(A+B).
represents how far a solution component is from ideal.
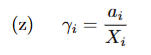
Activity Constant
products/reactants.

Classius Clapeyron
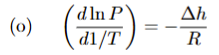
Compressibility Factor
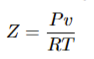
Definition of Activity
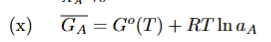
Expansion Coefficient
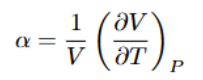
Gibbs-Helmholtz equation
COME BACK TO BRUHH
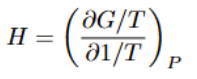
Gibbs Phase Rule
LEARN
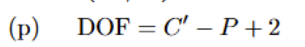
Henry’s Law, Raoult’s Law
Raoult’s is just as composition approaches 1, activity approaches just Xa.
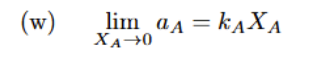
Isothermal Compressibility

Nurst Equation
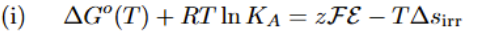
Single Particle Partition Function
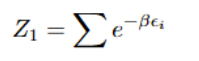
Van Der Waals EOS
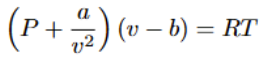
Regular Solution Model
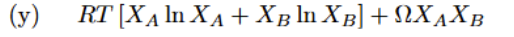
adiabatic, reversible, ideal gas, path?
PV^gamma = cst. When no heats exchanged, work comes from internally, so temperature drops when exerting work. vice versa. gamma = Cv/Cp, >1.
Entropy using boltzmann?
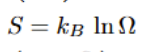
Gibbs Duhem
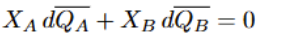
Partial Molar Enthalpy
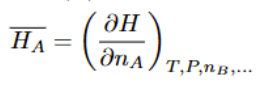
Chemical Potential
also = to activity
Standard Cell Potential
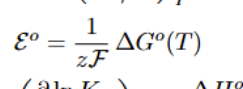
Uppercase vs lowercase (H vs h, V vs v?)
Uppercase is extensive, lowercase is molar specific.
Avg partial molar volume of water?
18 grams per mole
Aqueus Activity for Dissolving?
Activity referenced to molal of material you’re dissolving = gamma^(sum of stoich A+B ) * molality of component A]^(stoich A) * molality of component B^(stoich B)
Cell types?
Galvanic produces electricity, electrolysis uses energy.
3 galvanics:
daniel/displacement, formation, and concentration…