Chapter 7: Mole Concept and Stoichometry
1/13
Earn XP
Description and Tags
How are measurements made in chemistry?
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Mole
A term used to describe a quantity
Mole formula
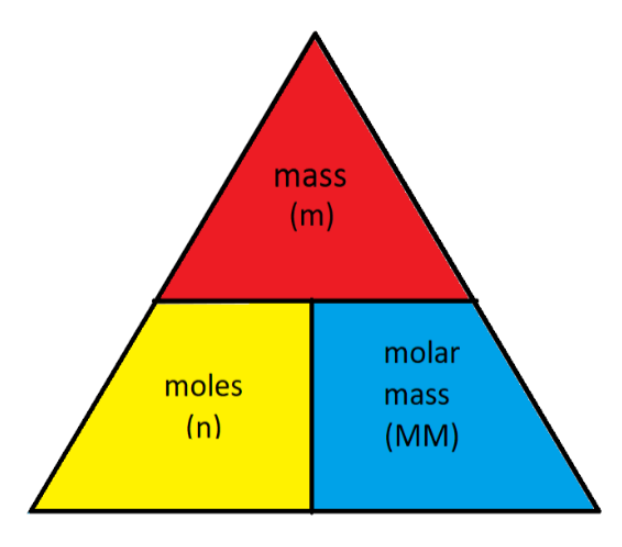
molar mass
mass of 1 moles of a substance
molar mass formula
adding the atomic mass of each element in a compound
moles to number of particles formula

stoichiometric ratio
Ratio of reactants and products in a chemical reaction
Steps for mole calcs with equations
Balance equation
Determine number of moles known
Use mole ratio
Work out moles of unknown
Calculate desired value from the question.
Percentage Composition
percentage (by mass) of an element present in a compound.
Steps for percentage ccomposition
Write out formula of a compound
Work out molar mass
Calculate mass of element specified.
Calculate percentage mass
Empirical formula
simplest whole number ratio of the elements contained in a compund
Steps to find Empirical Formula
Write the symbol of each element
Write the percentage composition and convert to a mass in 100g sample.
Convert masses to moles
Divide by the smallest number obtained
Write the empirical formula.
Limiting Reagent
The substance that is used up first in a chemical reaction OR the reactant that is completely consumed if the reaction goes to completion. Determines the amount of product produced and it is the reagent with the least number of moles.
Excess Reagent
The substance that is left over in a chemical reaction OR the reactant that has a proportion unreacted if the reaction goes to completion.
Limiting reagent steps
Balance equation
Calculate the number of moles of both reactants
Compare the stoichiometric and actual mole ratio
Determine the limiting reagent
Write the relationship between the limiting reagents and UNKNOWN
Calculate number of moles of UKNOWN
Convert moles to what the question asks.