Inorganic Qualitative Analysis
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
Group I: Mercury, Lead, Silver
MercuLeS
The insoluble chlorides
Group I
6M HCl
precipitant of insoluble chlorides
AgCl = white curdy precipitate
Ag + HCl
PbCl2 = white precipitate
Pb + HCl
Hg2Cl2 = white precipitate
Hg + HCl
Group II: Copper, Cadmium, Bismuth, Lead, Mercuric, Arsenic, Tin, Antimony
Copper-CaBLe MArTiAn
5% thioacetamide in diluted acid
precipitant of Group II
The acid insoluble sulfides
Group II
Copper, Cadmium, Bismuth, Lead
Group II A cations
Group IIA
insoluble in sodium sulfide (Na2S)
Mercuric, Arsenic, Tin, Antimony
Group IIB cations
Group IIB
soluble in sodium sulfide (Na2S)
Group III: Nickel, Cobalt, Zinc, Chromium, Iron, Manganese
NiCo ZaCh Iron Man
5% thioacetamide in ammonia (NH4)
precipitant of group III
The base insoluble sulfides
Group III
Nickel, Cobalt
Group IIIA cations
Group IIIA
soluble in 1M HCl
Group IIIB
insoluble in 1M HCl
Yellow precipitated sulfides
Cd2+
Sn2+ (brown)
As2+
As5+
Orange precipitated sulfides
Sb2+
Sb5+ (red-orange)
White precipitated sulfide
Zn2+
Pink precipitated sulfide
Mn2+
Black precipitated sulfide
Cu2+
Bi3+
Pb2+
Sn4+
Hg2+
Co2+
Ni2+
Ag+
Fe2+
Group IV: Barium, Calcium, Strontium
BaCal St
Ammonium carbonate (NH4)2CO3 + 95% ethanol
precipitant of group IV
The soluble sulfides
The insoluble carbonates
Group IV
All are white precipitates
Group IV precipitates
Group V: Potassium, Lithium, Sodium, Magnesium, Ammonium
PoLiS Magnum Ammo
None
precipitant for group V
The soluble group
Group V
Group V
yield colorless solution for all major identification test reagents
HCl + Ammonium hydroxide
confirmatory reagent for group I
Ag+
white curdy precipitate with HCl, soluble in NH4OH
Pb+2
white precipitate with HCl, insoluble in NH4OH
Potassium ferrocyanide
confirmatory reagent for group II
Brownish red precipitate
copper + potassium ferrocyanide
Yellowish white precipitate
bismuth + potassium ferrocyanide
Dimethylglyoxime (DMG)
confirmatory reagent for group III
Colorless urine
zinc + DMG
Cherry red precipitate
nickel + DMG
Orange solution
cobalt + DMG
Saturated calcium sulfate (CaSO4)
confirmatory reagent for group IV
White precipitate
calcium + saturated CaSO4
Colorless solution
strontium + saturated CaSO4
Diluted NaOH
confirmatory reagent for group 5
Odorless
potassium + diluted NaOH
Pungent odor
ammonium + diluted NaOH
Copper
preliminary examination: blue salts
Ni+2
Ferrous (Fe+2)
Chromic (Cr+3)
Manganates
preliminary examination: green
Chromate
Ferric ion (Fe+3)
preliminary examination: yellow
Dichromate ion
preliminary examination: red-orange
Permanganate
preliminary examination: purple
Mn+2
Cobaltous (Co+2)
preliminary examination: pink
Flame test
an analytical method used for detecting the presence of certain metals where a small amount of a substance is volatilized and the characteristic glow of the substance is observed
Yellow
luminous flame
Blue flame
non luminous flame; where the flame test is conducted
Upper oxidizing zone
Upper reducing zone
Hottest portion of the flame
Lower oxidizing zone
Lower reducing zone
Lower temperature zone
Parts of flame
(Top to bottom)
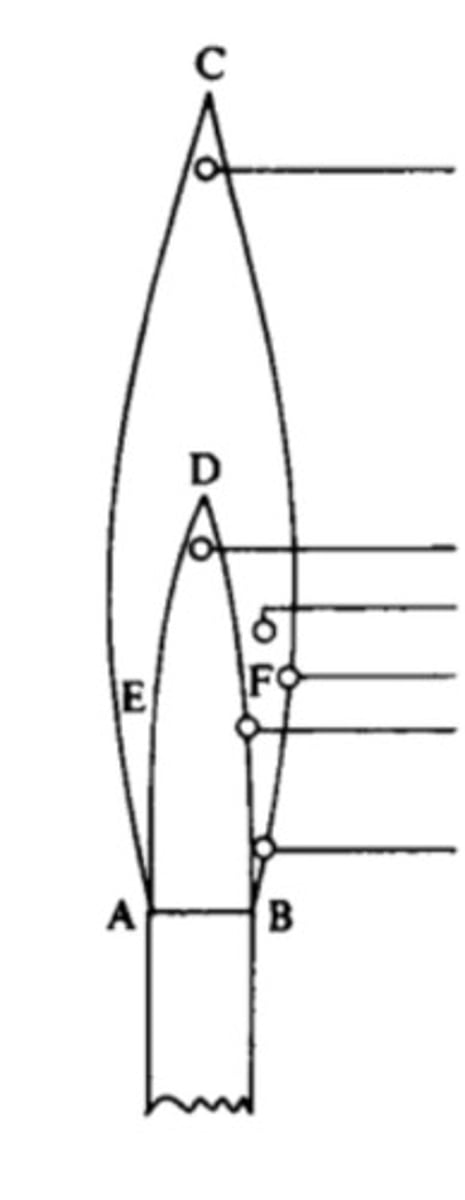
1/3 height of the flame
hottest portion of the flame; where the flame test is conducted
Na
persistent golden yellow in normal flame test
K
violet (lilac) in normal flame test
Lithium
Carmine red in normal flame test
Strontium
Crimson red in normal flame test
Calcium
brick red in normal flame test
Barium
yellowish green in normal flame test
Borates, Thallium, Copper
green in normal flame test
Lead, Arsenic, Bismuth, Antimony, Copper
blue (wire slowly corroded) in normal flame test
Flame test with cobalt glass
done using platinum wire dipped in conc HCL over non-luminous bunsen flame
the cobalt glass absorbs radiation form Na
Nil
Na in flame test with cobalt glass
Crimson red
K in flame test with cobalt glass
Light green
Ca in flame test with cobalt glass
Purple
Sr in flame test with cobalt glass
Bluish green
Barium in flame test with cobalt glass