Kaplan MCAT General Chemistry Chapter 4: Compounds and Stoichiometry
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
Compounds
Substances composed of two or more elements in a fixed proportion.
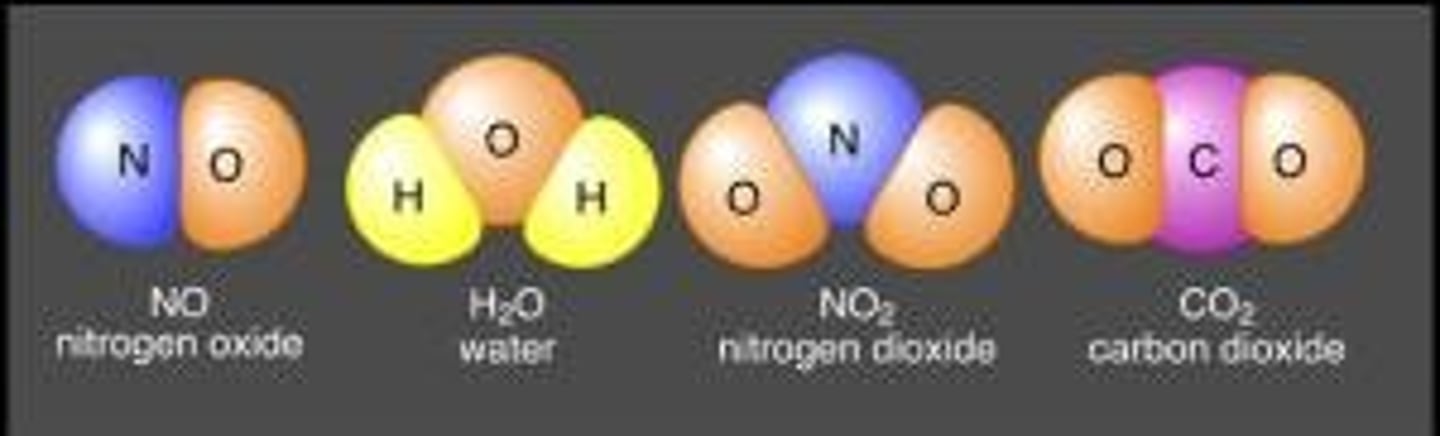
Molecular weight
The mass (in amu) of the constituent atoms in a compound as indicated by the molecular formula.
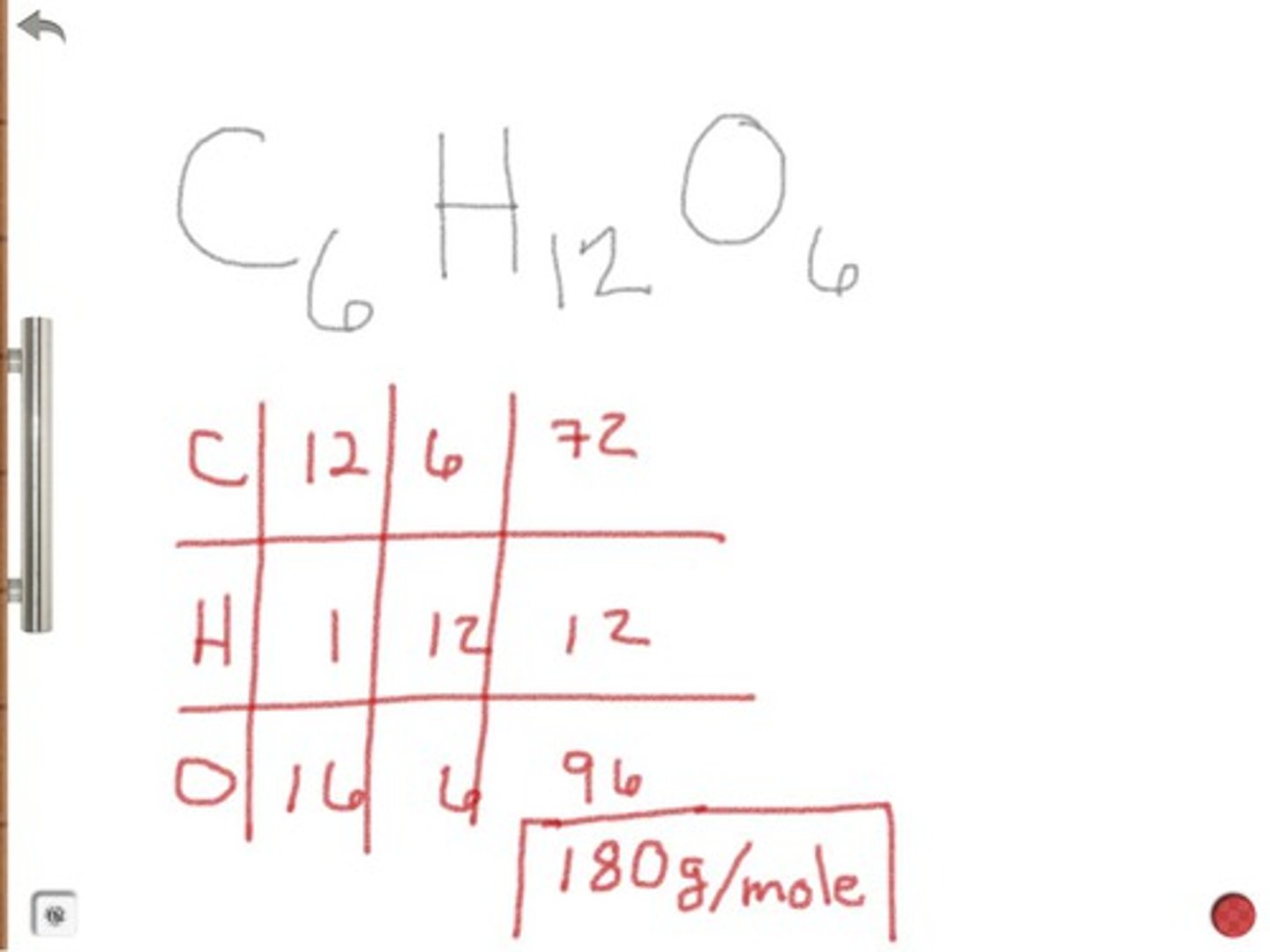
Moles from mass equation:

How to find moles?
# moles= mass in grams/molecular weight
Molar mass
The mass of one mole (Avogadro's number 6.023x10^23 particles) of a compound; usually measured in grams per mole.
Gram equivalent weight
A measure of the mass of a substance that can donate one equivalent of the species of interest.
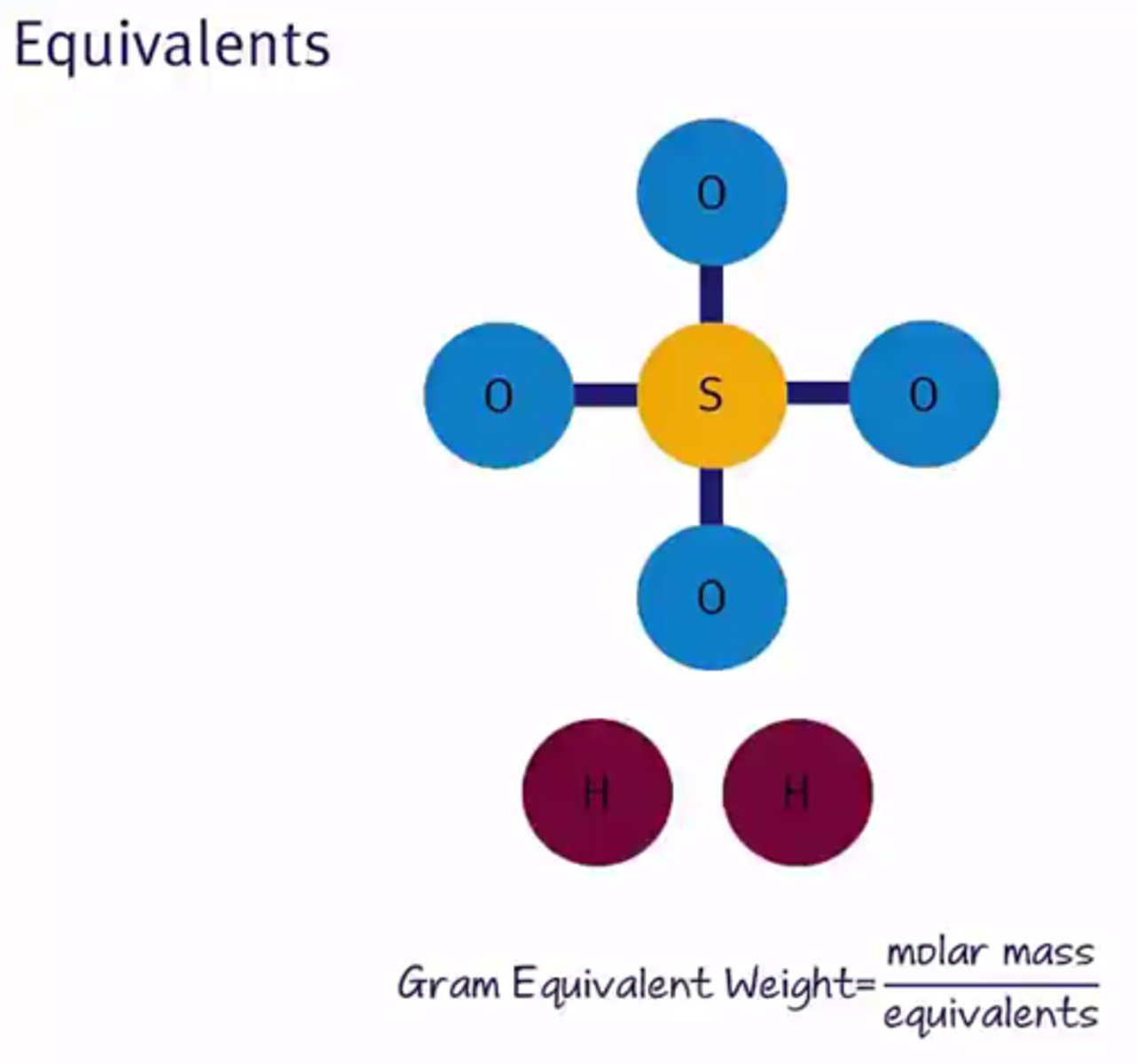
Gram equivalent weight equation:
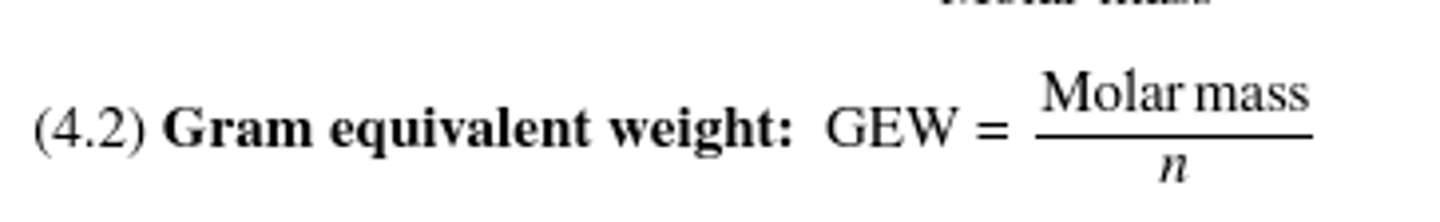
Normality
The ratio of equivalents per liter; it is related to molarity by multiplying the molarity by the number of equivalents present in one mole of a compound.
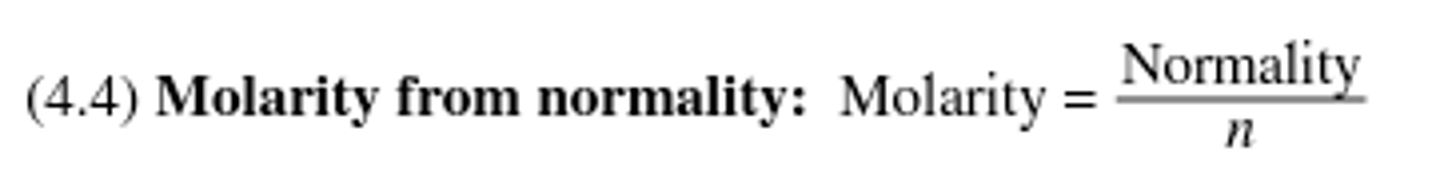
Equivalents
Moles of the species of interest, equivalents are most often seen in acid-base chemistry ( H+ ions or -OH ions) and oxidation-reduction reactions.
Equivalents from mass equation:
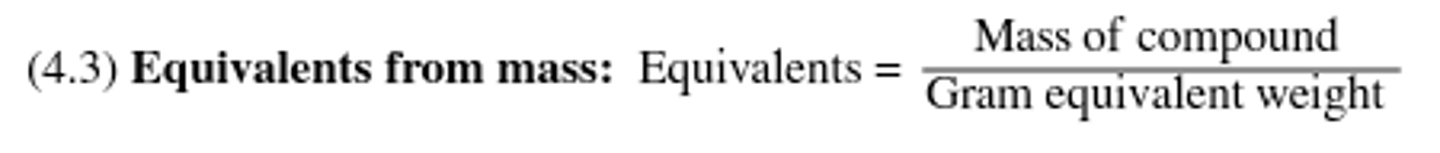
Molarity from normality equation:
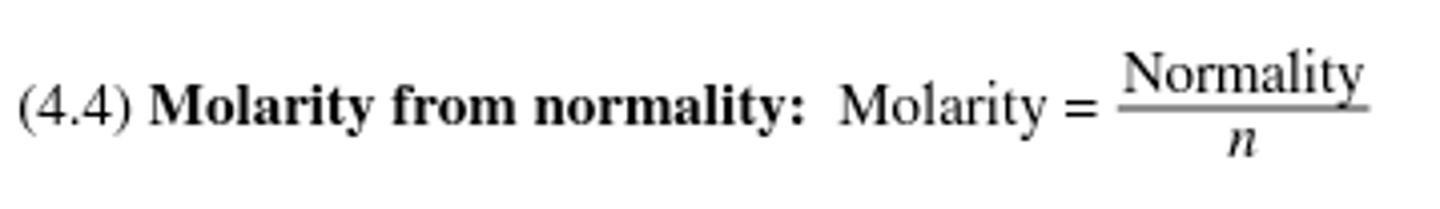
The law of constant composition
any pure sample of a compound will contain the same elements in the same mass ratio.
Empirical formula
The smallest whole number ratio of the elements in a compound.
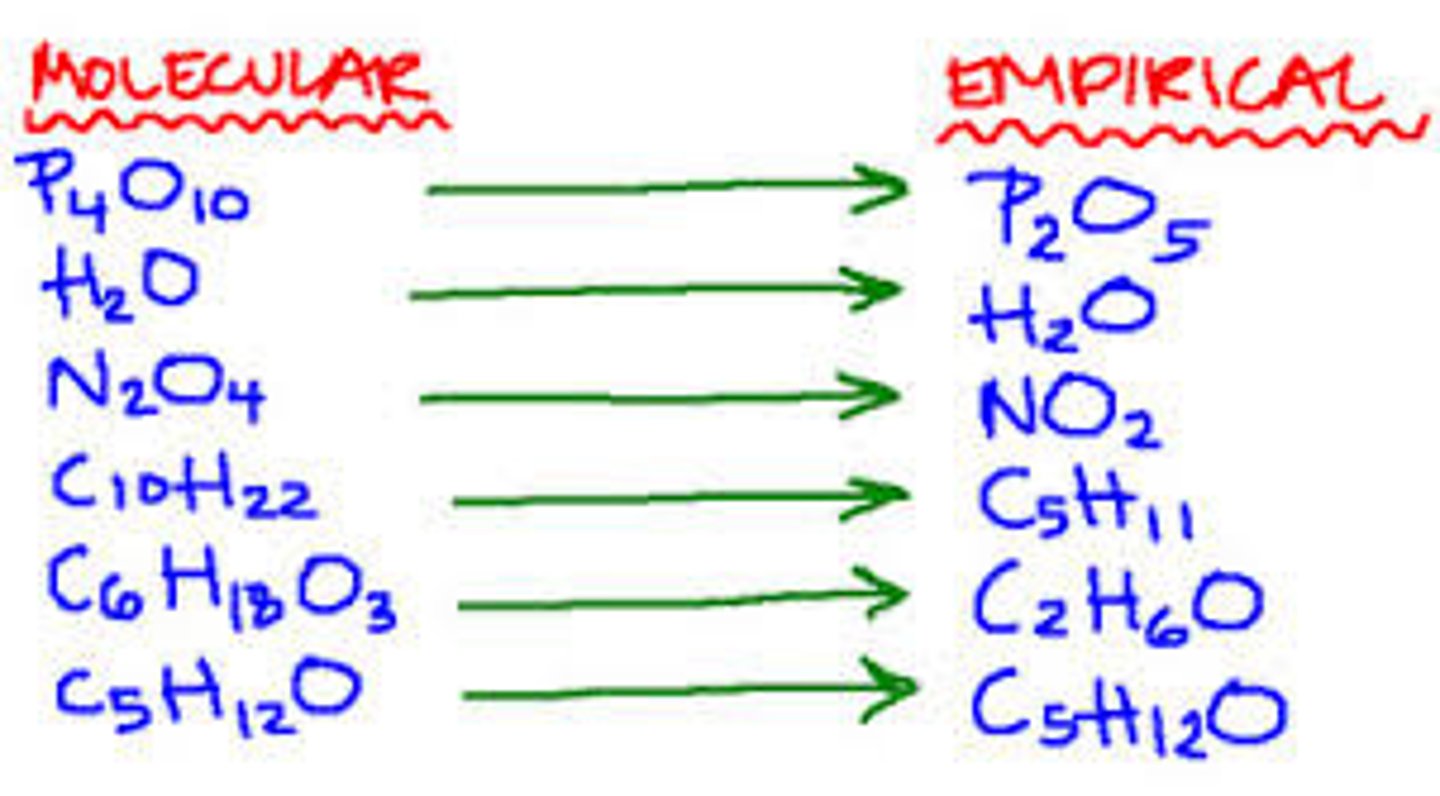
Molecular formula
Either the same as the empirical formula or a multiple, it gives the exact number of atoms of each element in a compound.
Percent composition equation:
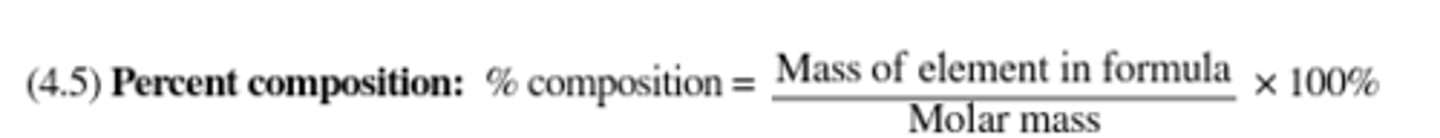
Types of chemical reactions?
1) Combination reaction
2) Decomposition reaction
3) Displacement reaction - single or double
4) Neutralization reaction
5) Combustion
Combination reaction
When two or more reactants combine to form one product.
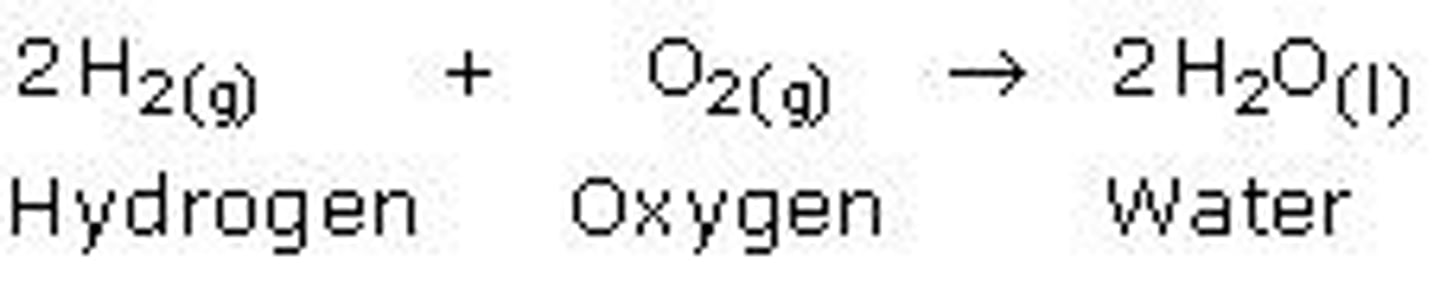
Decomposition reaction
When one reactant is chemically broken down into two or more products.

Single displacement reaction
Occur when an ion of one compound is replaced by another element.
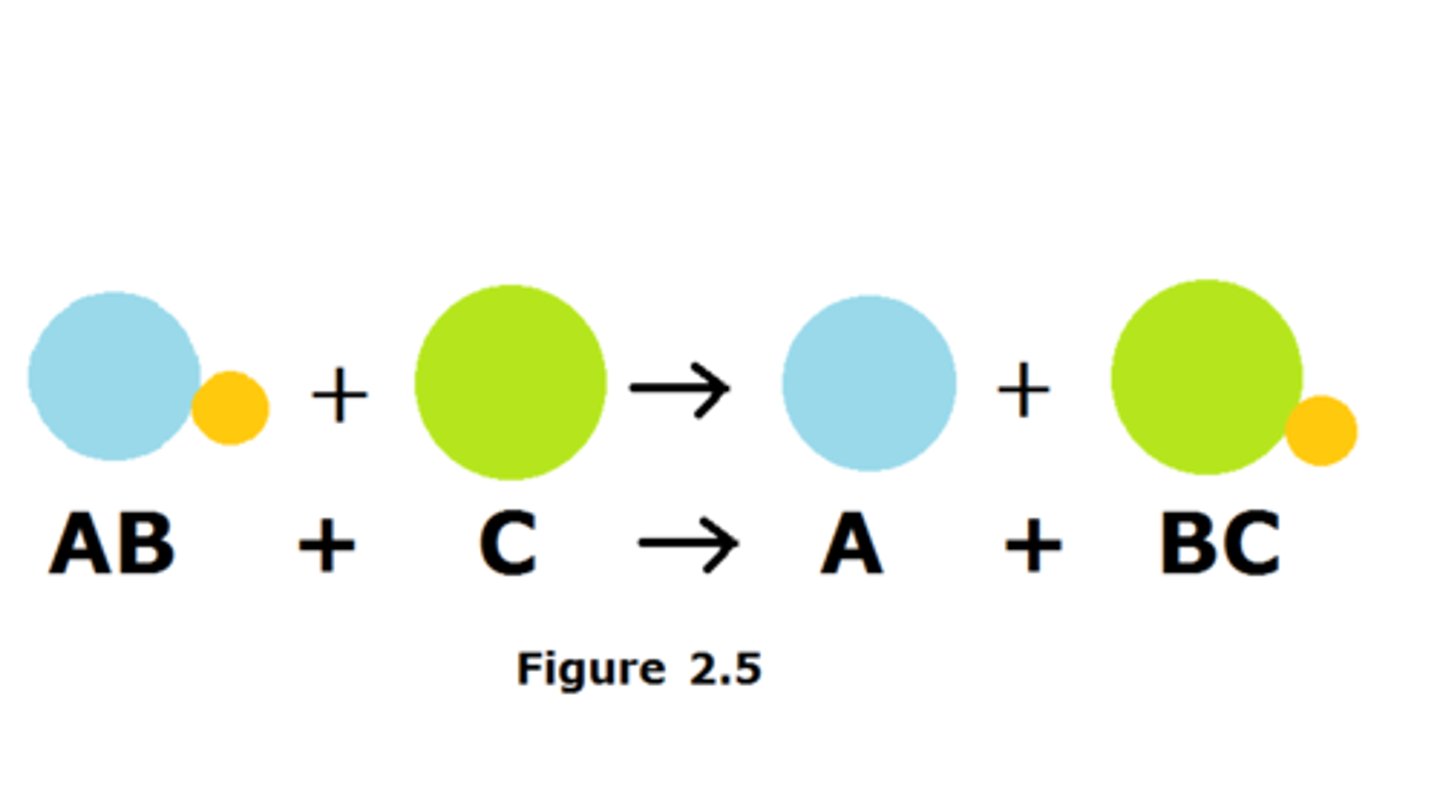
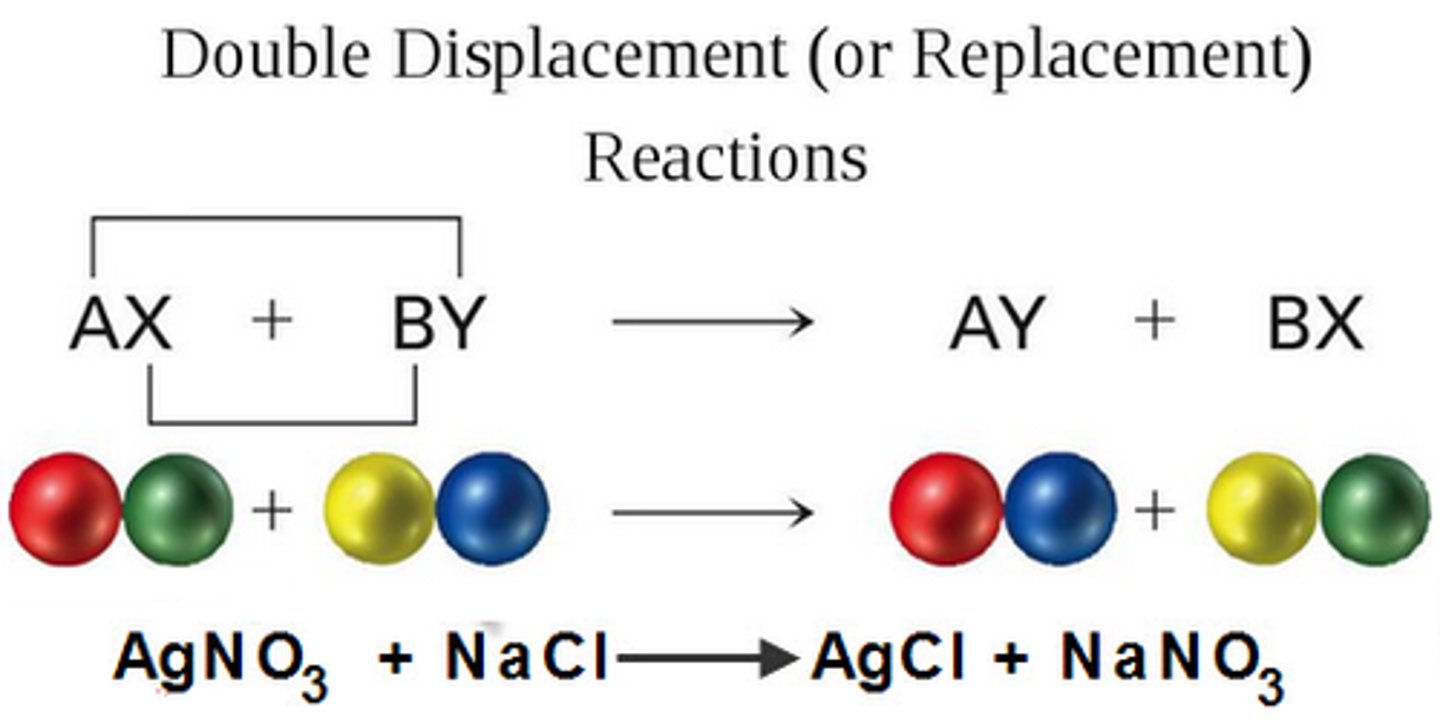
Double-displacement reaction
When elements from two different compounds trade places with each other to form two new compounds.
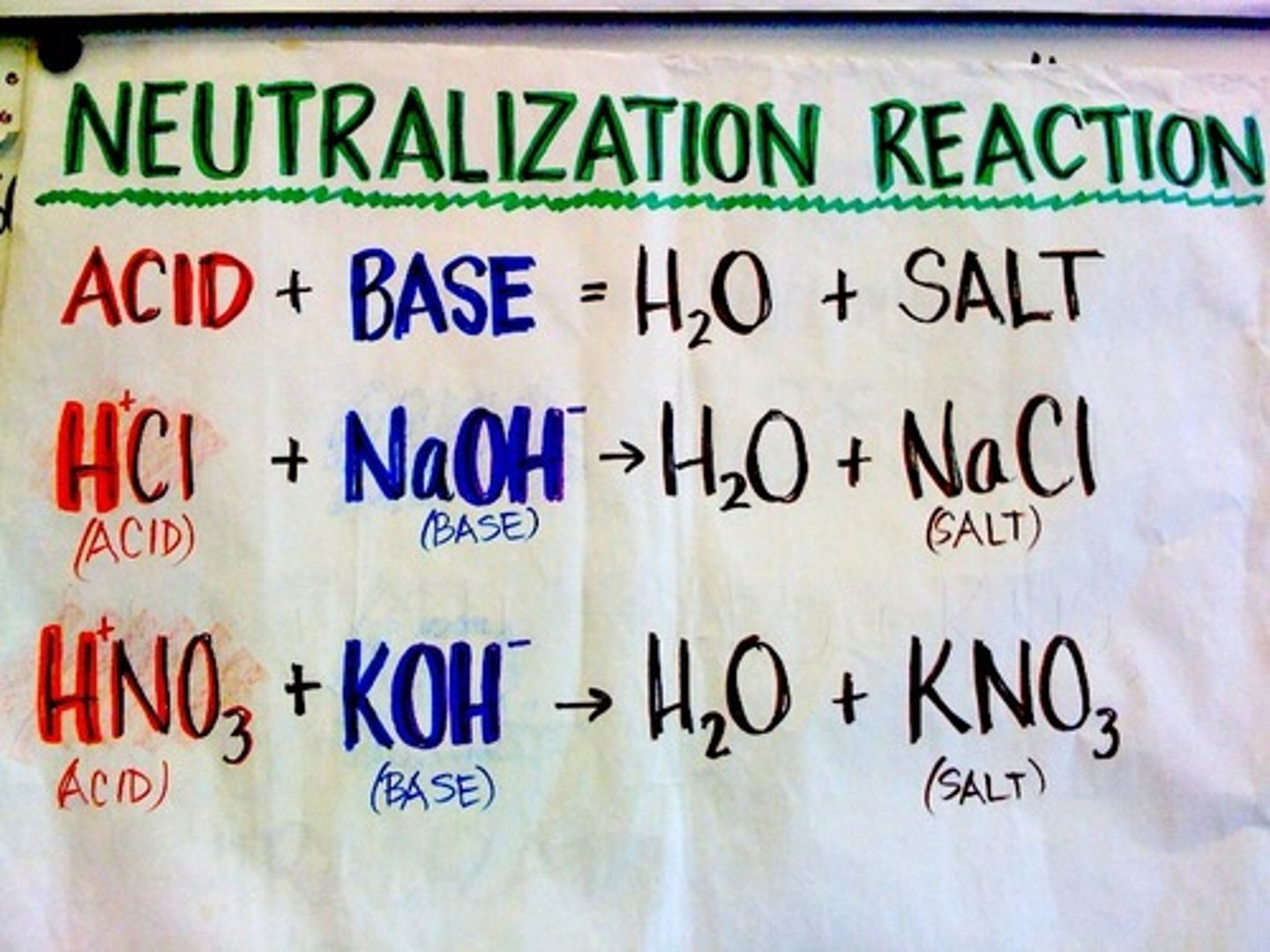
Neutralization reaction
double-displacement reaction in which an acid reacts with a base to form a salt (and, usually, water)
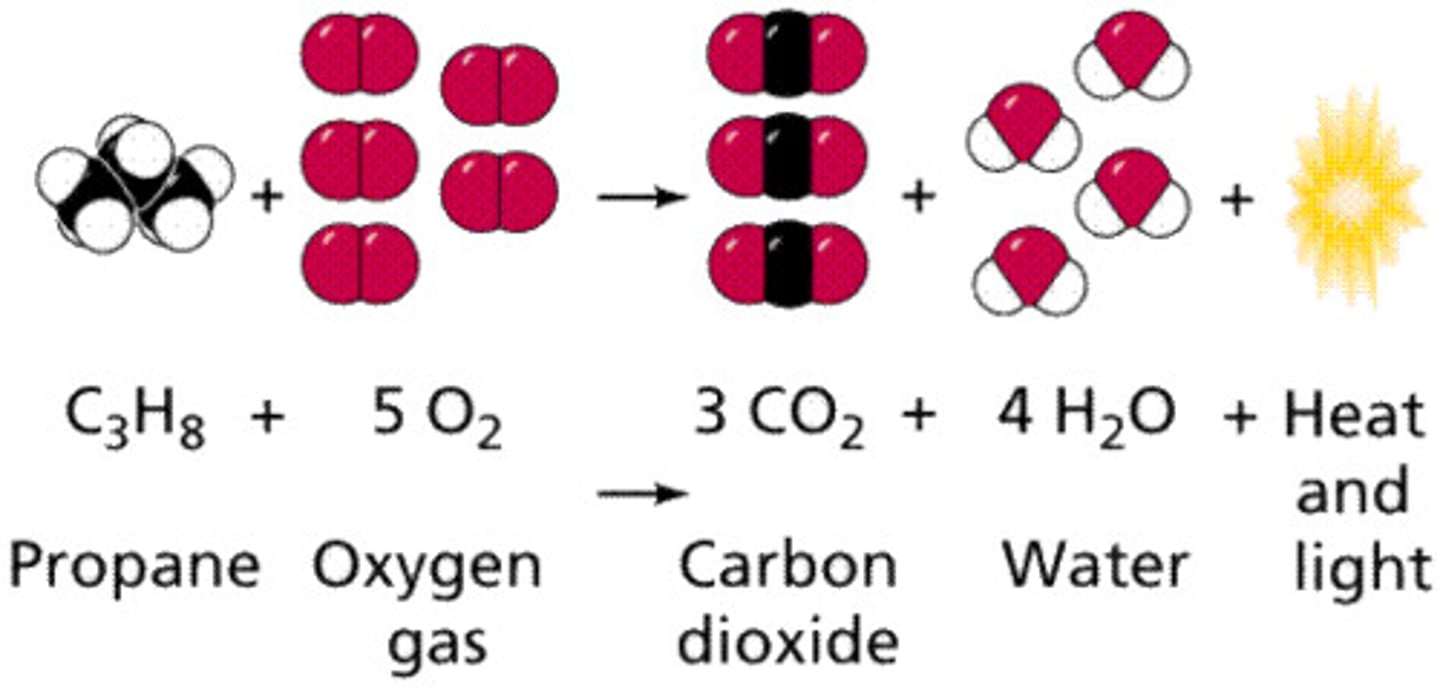
Combustion reaction
Occurs when a fuel and an oxidant (typically oxygen) react, forming the products water and carbon dioxide ( If the fuel is a hydrocarbon).
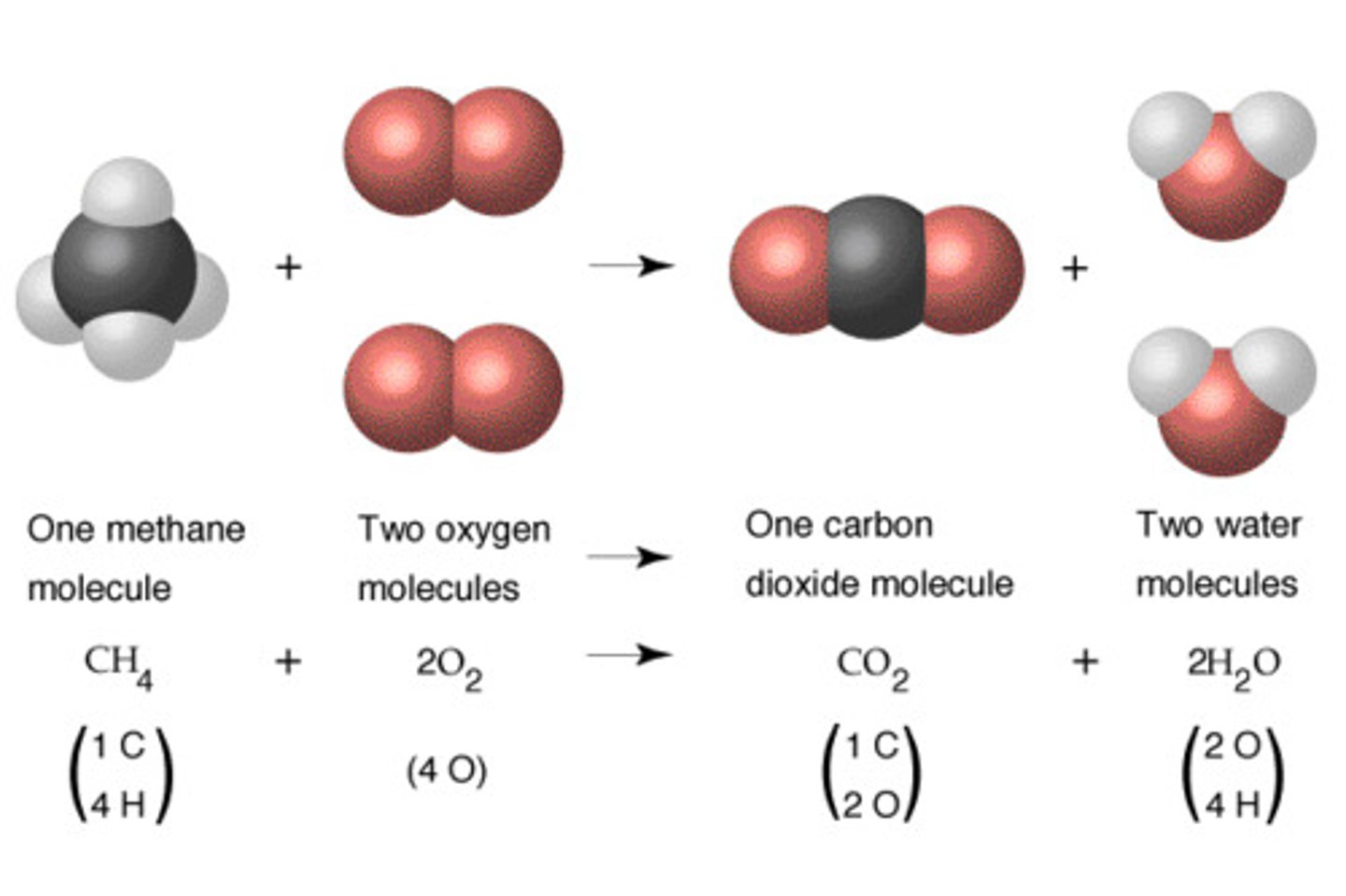
Balancing chemical equations:
1) balance least common atoms
2) Balance more common atoms ( usually H or O)
3) Balance charge (if necessary)
1 mole of any ideal gas at STP
22.4 L
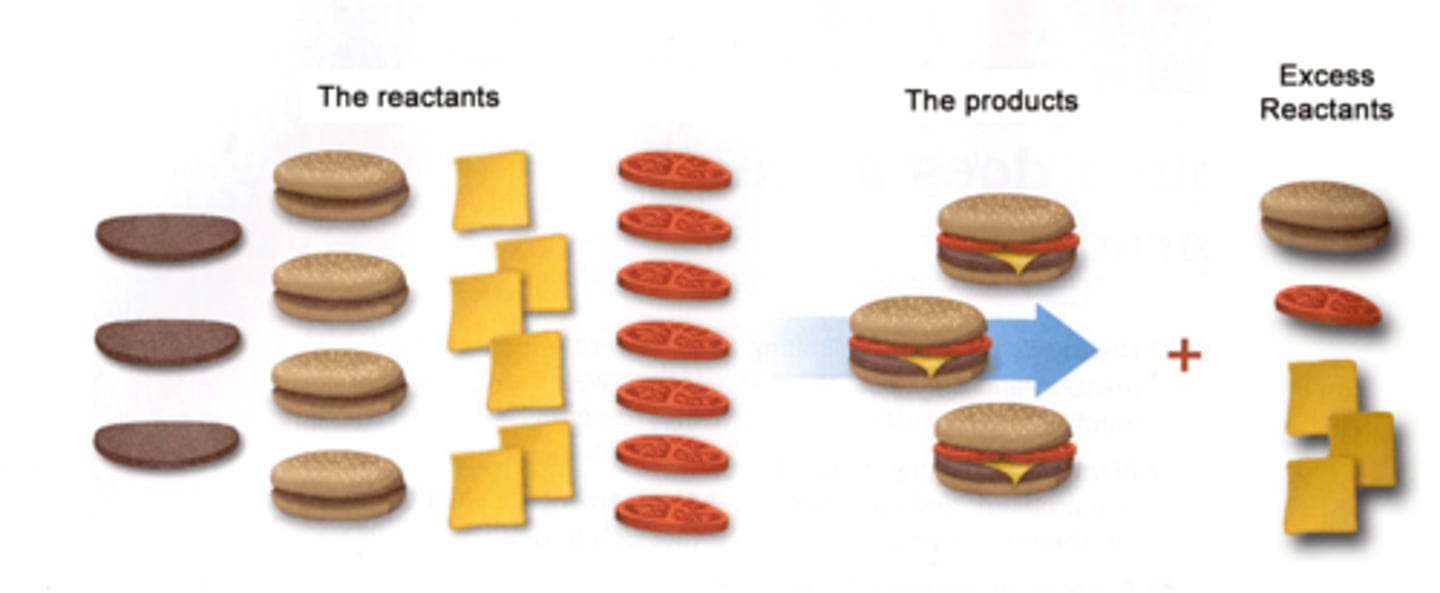
Limiting reagent
The reactant that will be consumed first in a chemical reaction. Determines the amount of product that can be formed.
all comparisons must be in moles
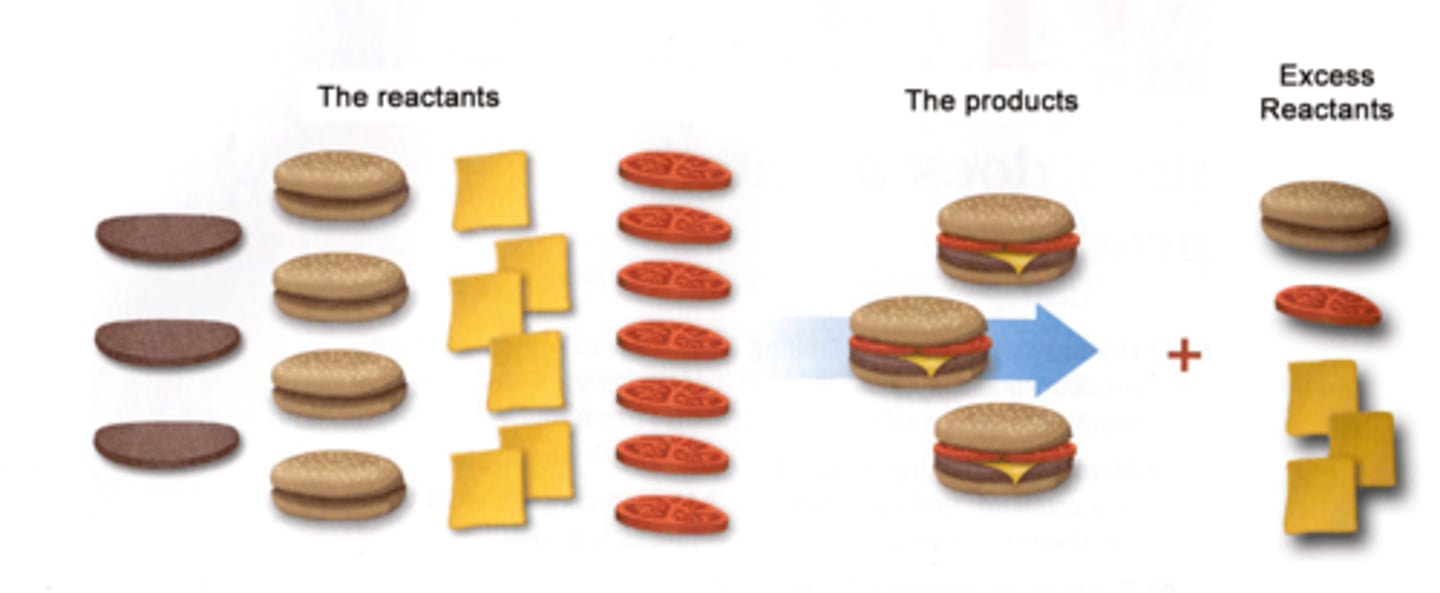
Excessive reagents
The reagents left over after limiting reagent is consumed.
Theoretical yield
The amount of product generated if all of the limiting reactant is consumed with no side reactions.
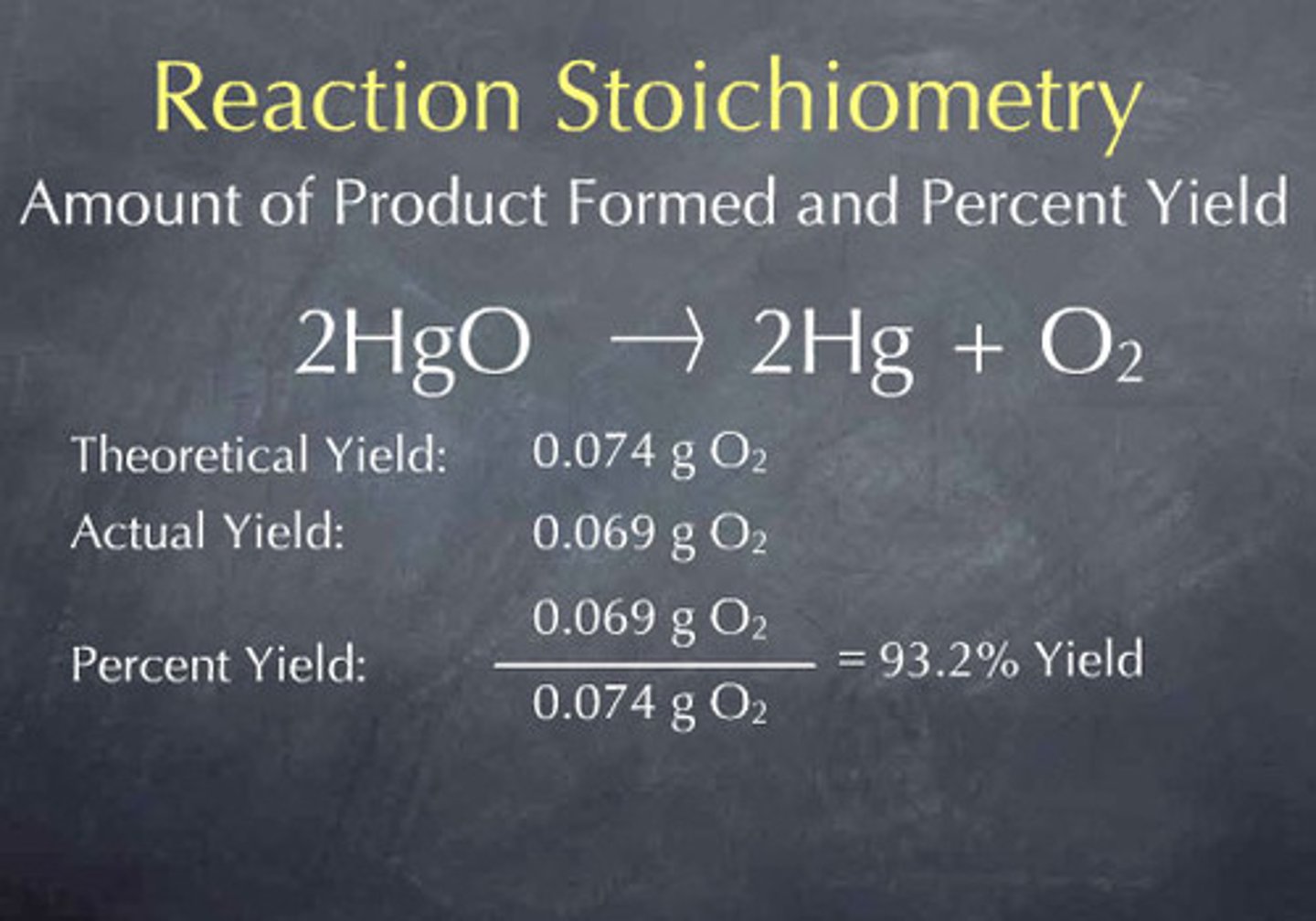
Actual yield
What you actually get; typically lower than theoretical yield.
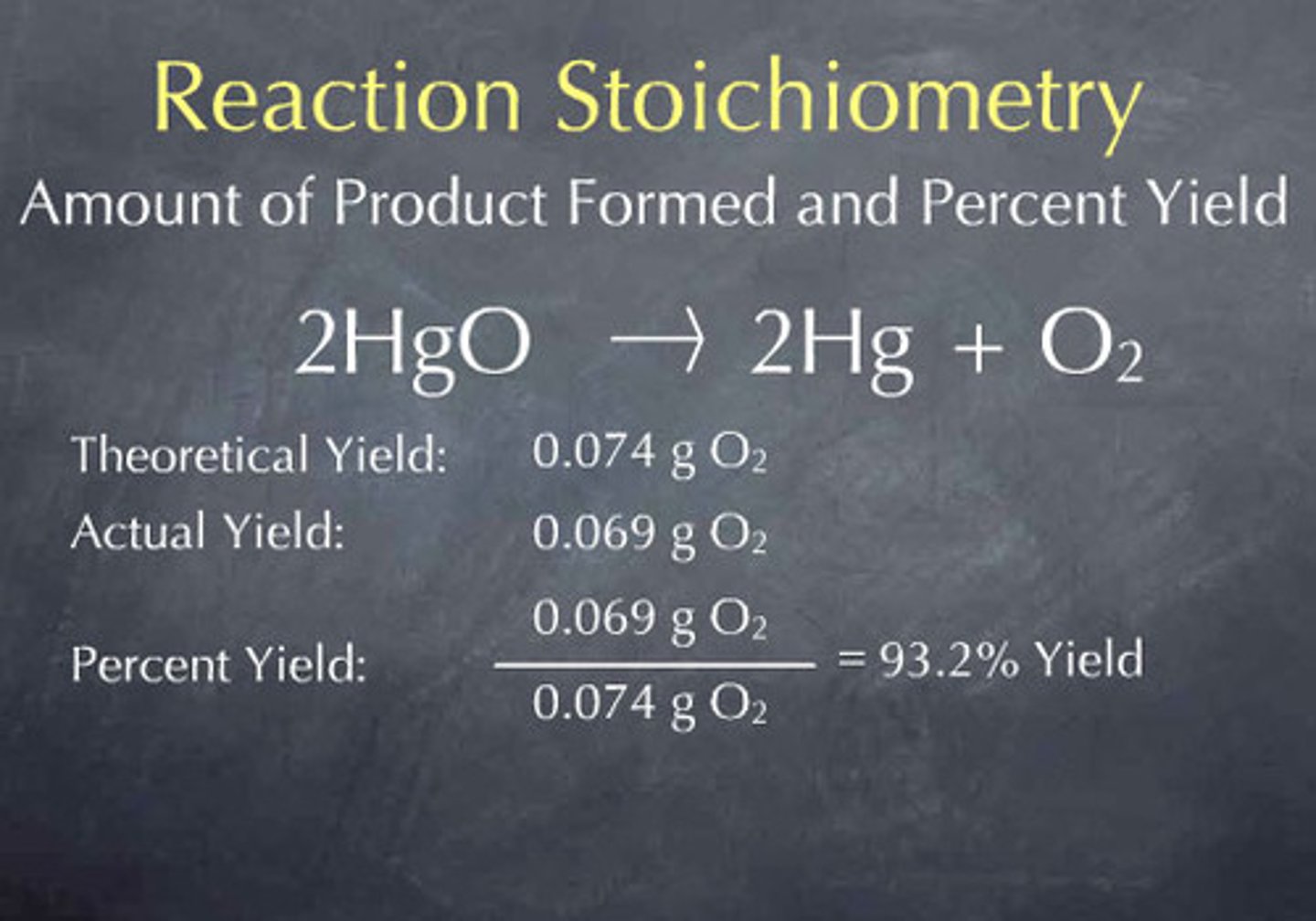
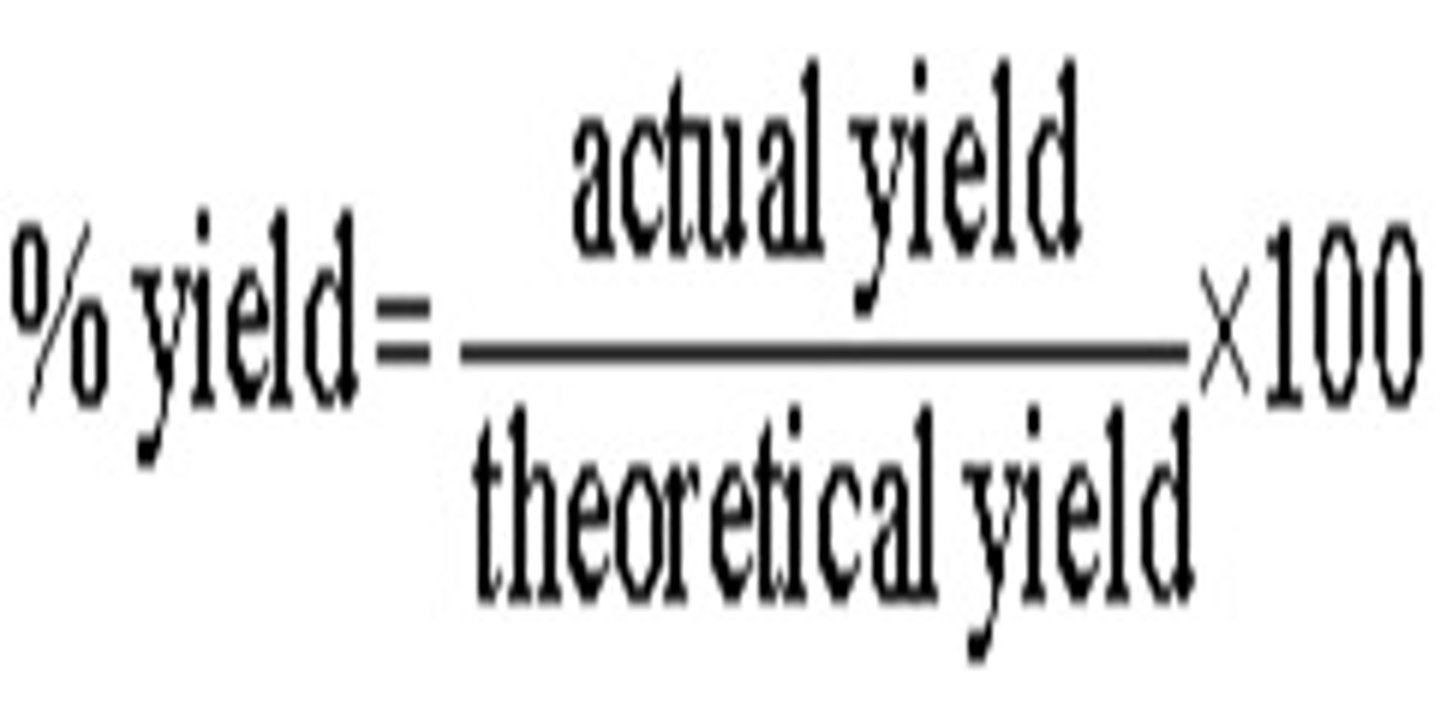
Percent yield
Calculated by dividing actual yield by theoretical yield and multiplying by 100.
Ionic compound nomenclature:
What does the roman numeral in Fe (II) mean?
Roman numeral denotes the ionic charge on atoms that posses more than one ionic state.
I.e., Fe(II) = Fe2+ vs Fe(III) = Fe3+
Monoatomic ions named?
Monoatomic ions are named by dropping the ending of the name of the element and then adding -ide
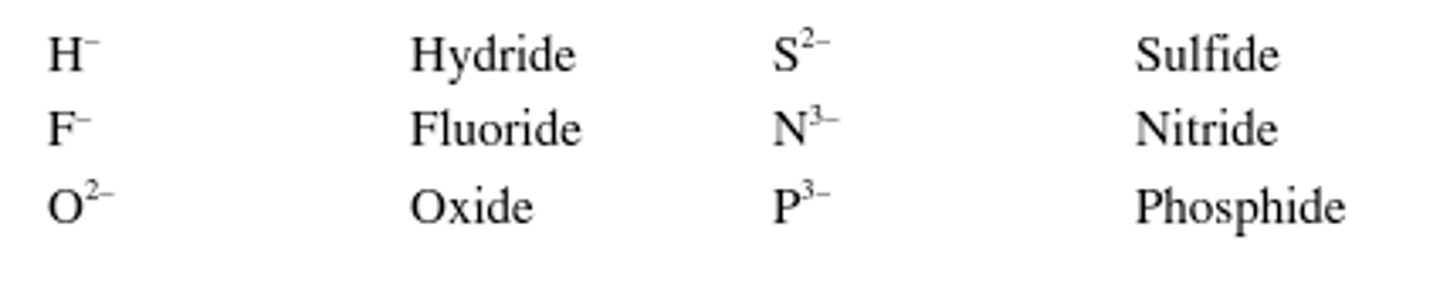
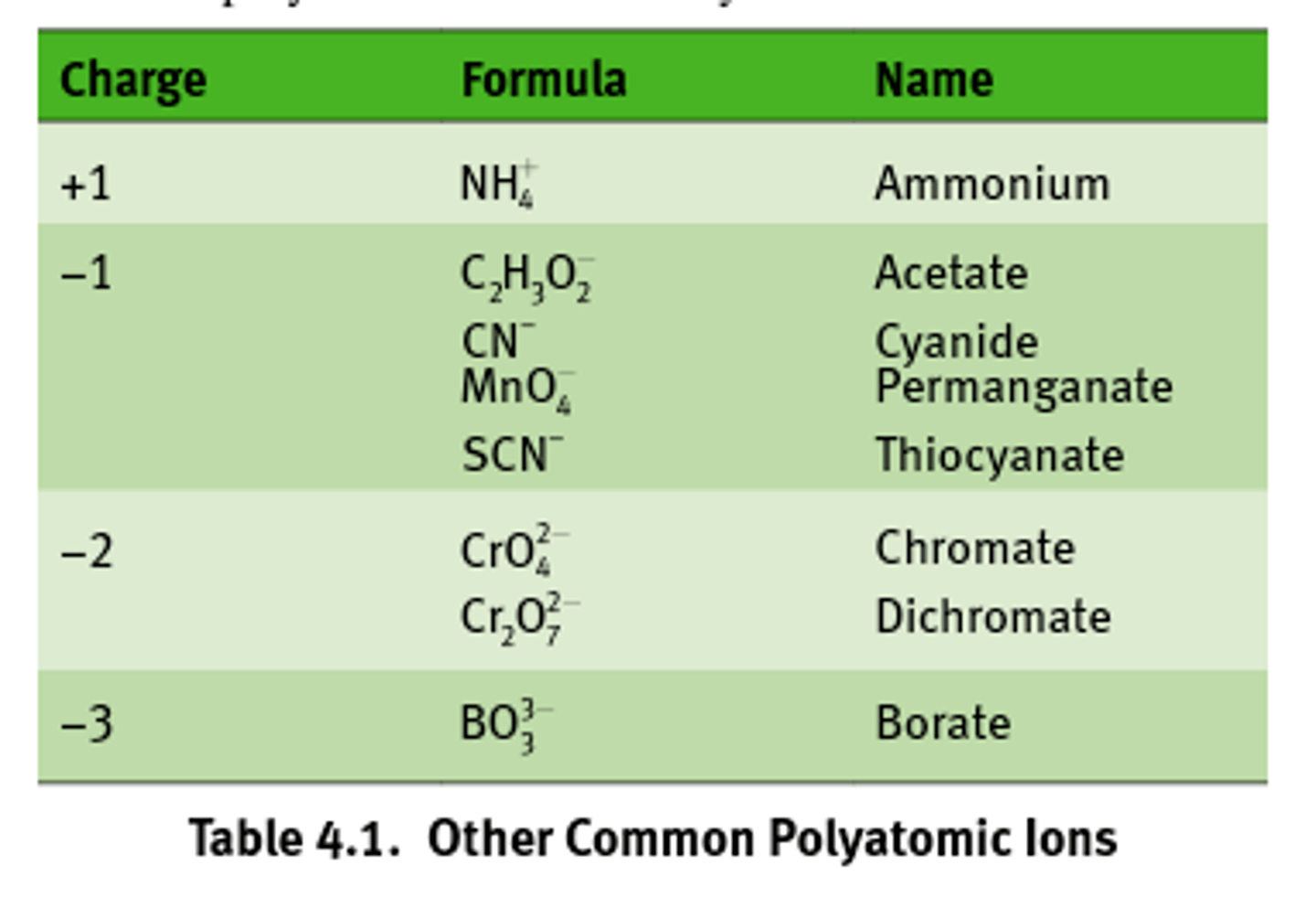
Polyatomic anions named?
Many polyatomic anions contain oxygen and are called oxyanions.
When an element forms two oxyanions, the smaller - ite and the larger = ate.

Multiple oxyanions?
If an extended series of oxyanions, Hypo goes first and per goes last.

Polyatomic anions with H+?
Polyatomic anions often gain one or more H+. The resulting ions are named by adding hydrogen or dihydrogen in front of the anion's name.

Mnemonic for -ite vs -ate?
The l-IGHT-est anions have the fewest oxygens, the heaviest anions ATE the most oxygens.
Common polyatomic ions to know:
Ionic charge
Predictable by group number and the type of element (metal vs nonmetal) for respective elements.
What charges will metals form?
Metals for positively charged cations based on group number.
What change will nonmetals form?
Nonmetals form negatively charged anions based on the number of the electrons needed to achieve the octet.
Electrolytes
solutes that enable solutions to carry current
Contain equivalents of ions from molecules that dissociate in solution. The strength of an electrolyte depends on the degree of solvation
ionic compounds are good electrolytes because they dissolve readily
Solvation
the process by which the positive and negative ions of an ionic solid become surrounded by solvent molecules
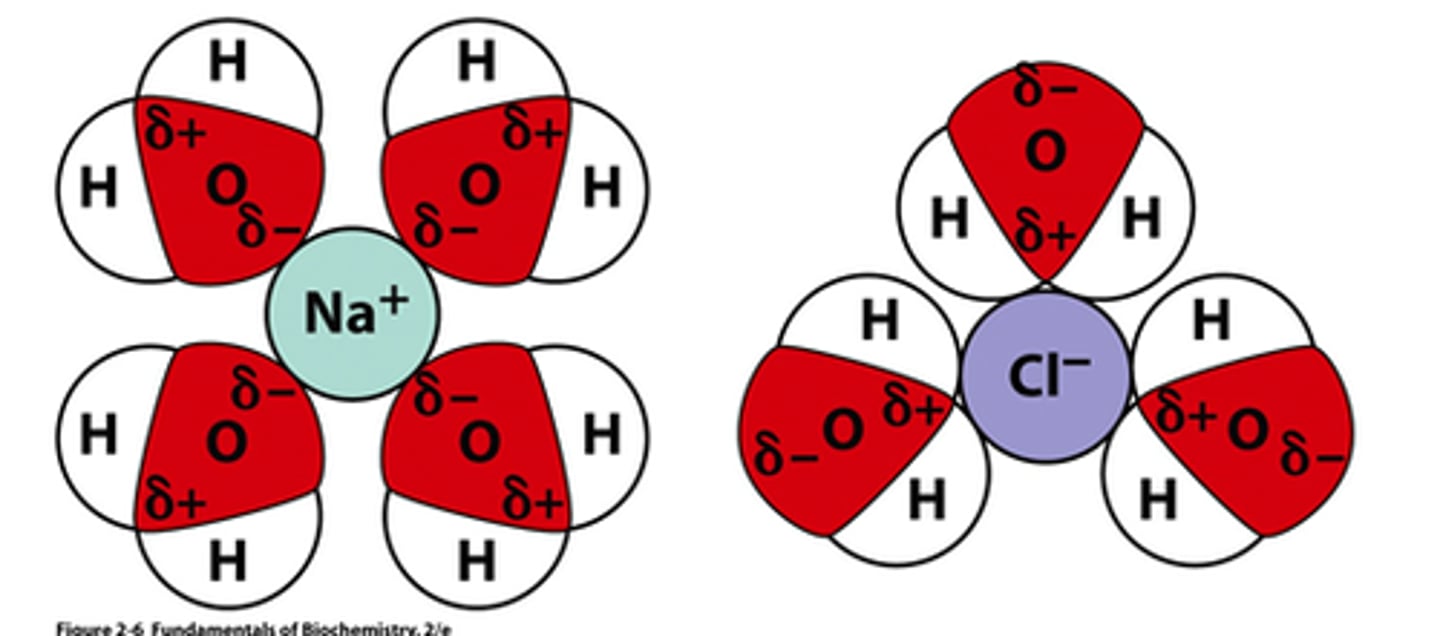
ionic compounds are measured with
formula weights, not molecular weights like covalent compounds