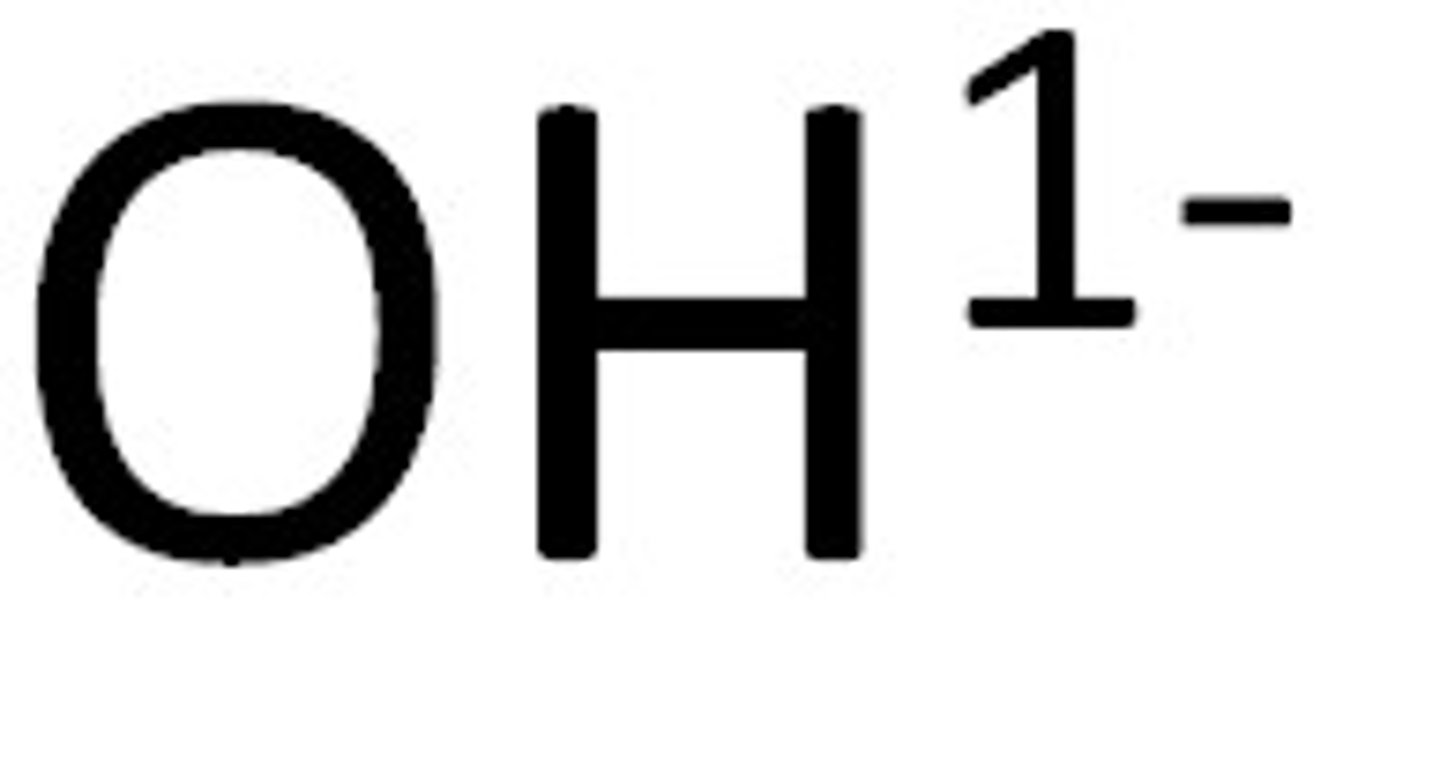Solubility Rules
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
Li+
Soluble with anything (alkali metal)
Na+
Soluble with anything (alkali metal)
K+
Soluble with anything (alkali metal)
Rb+
Soluble with anything (alkali metal)
Cs+
Soluble with anything (alkali metal)
Ammonium
Soluble with anything
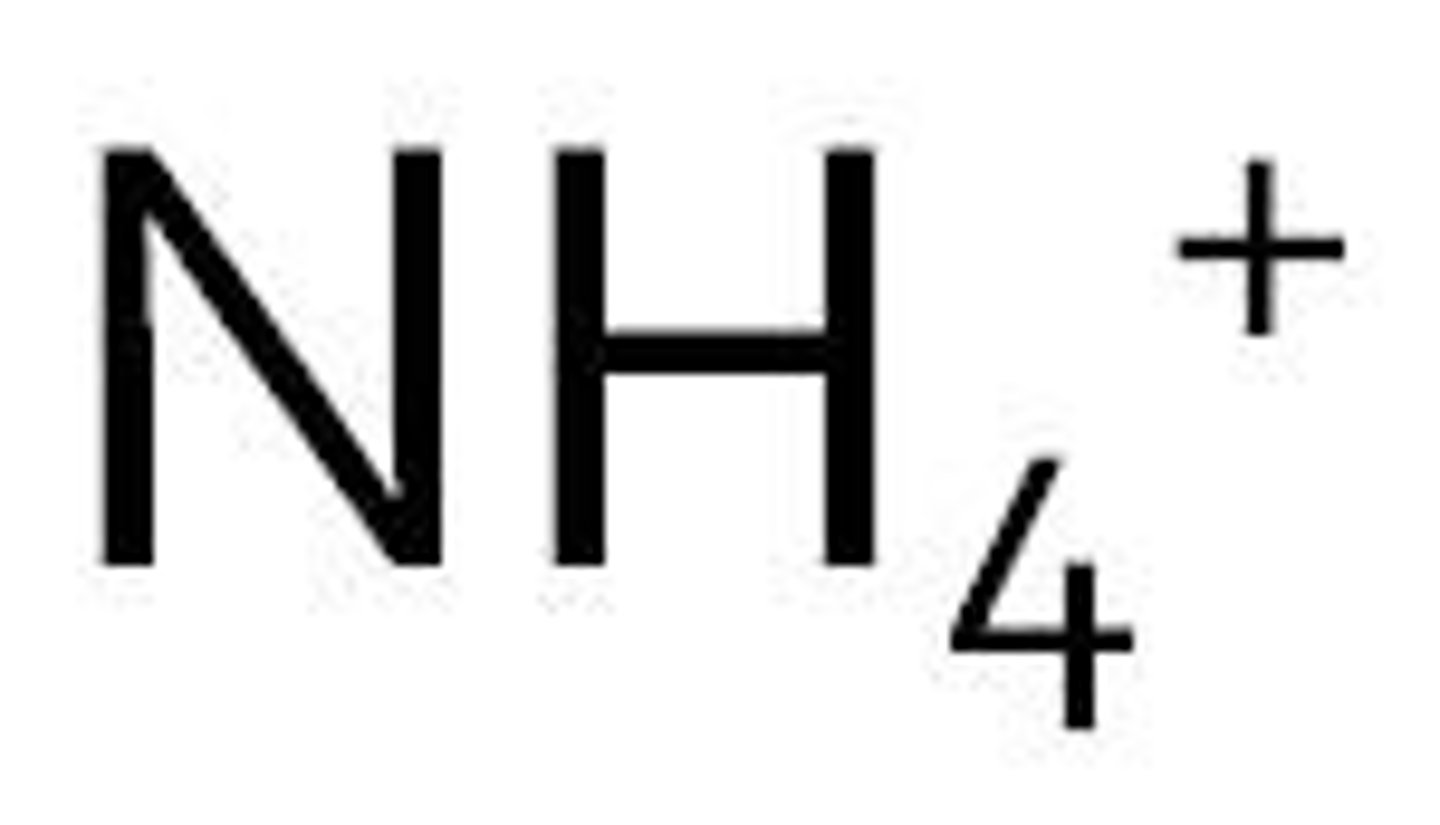
Nitrate
Soluble
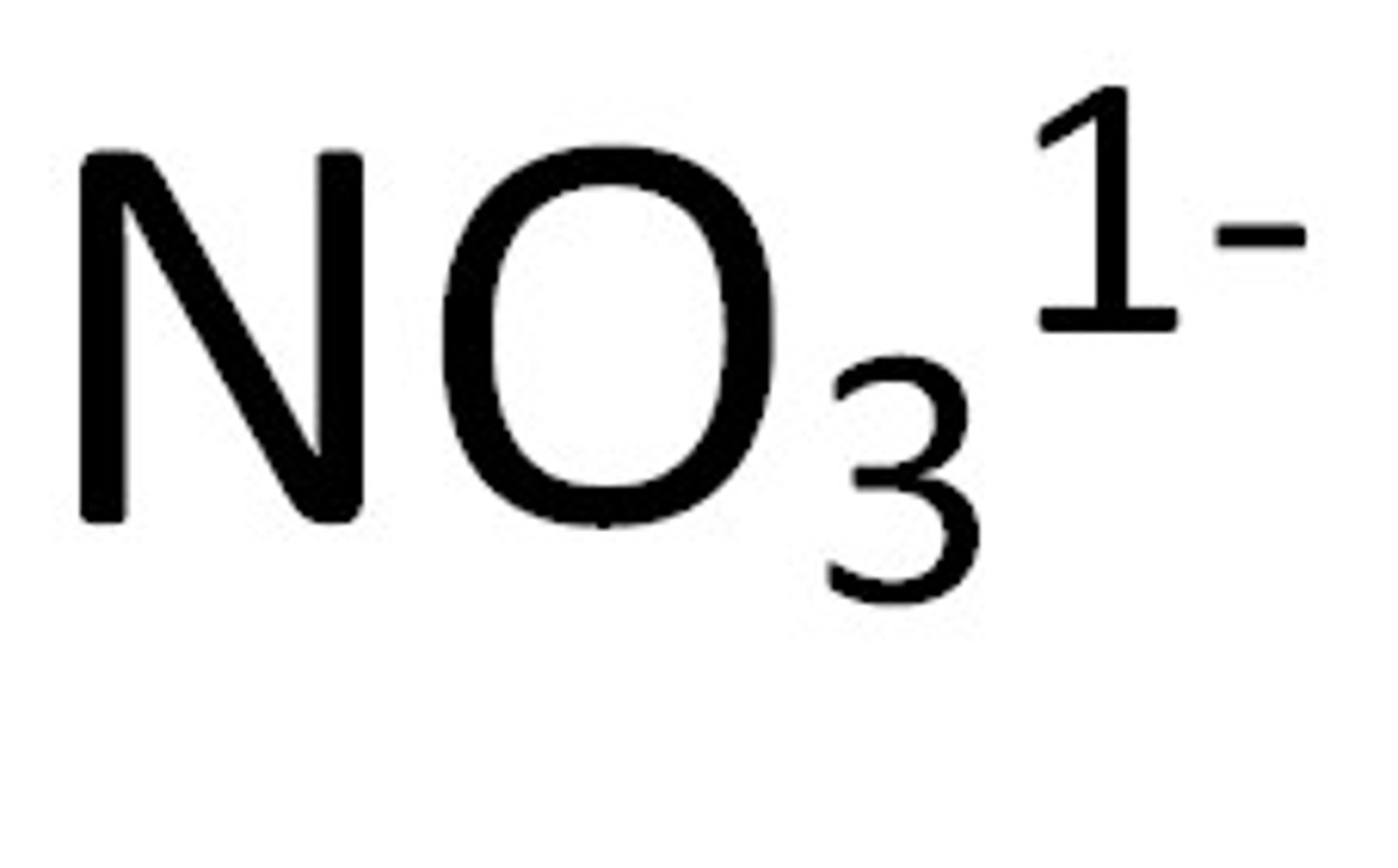
Bicarbonate/ Hydrogencarbonate
Soluble
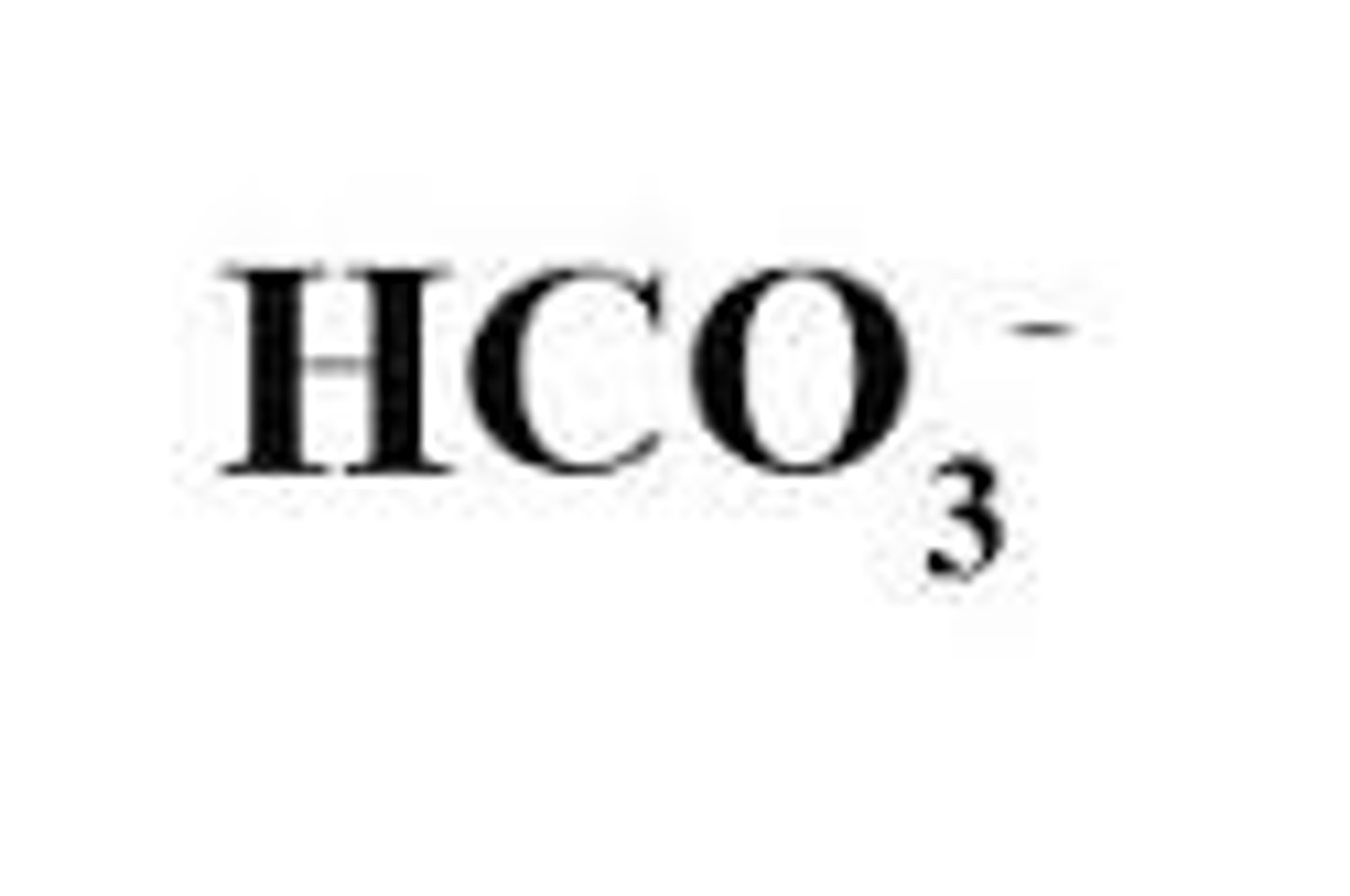
Chlorate
Soluble
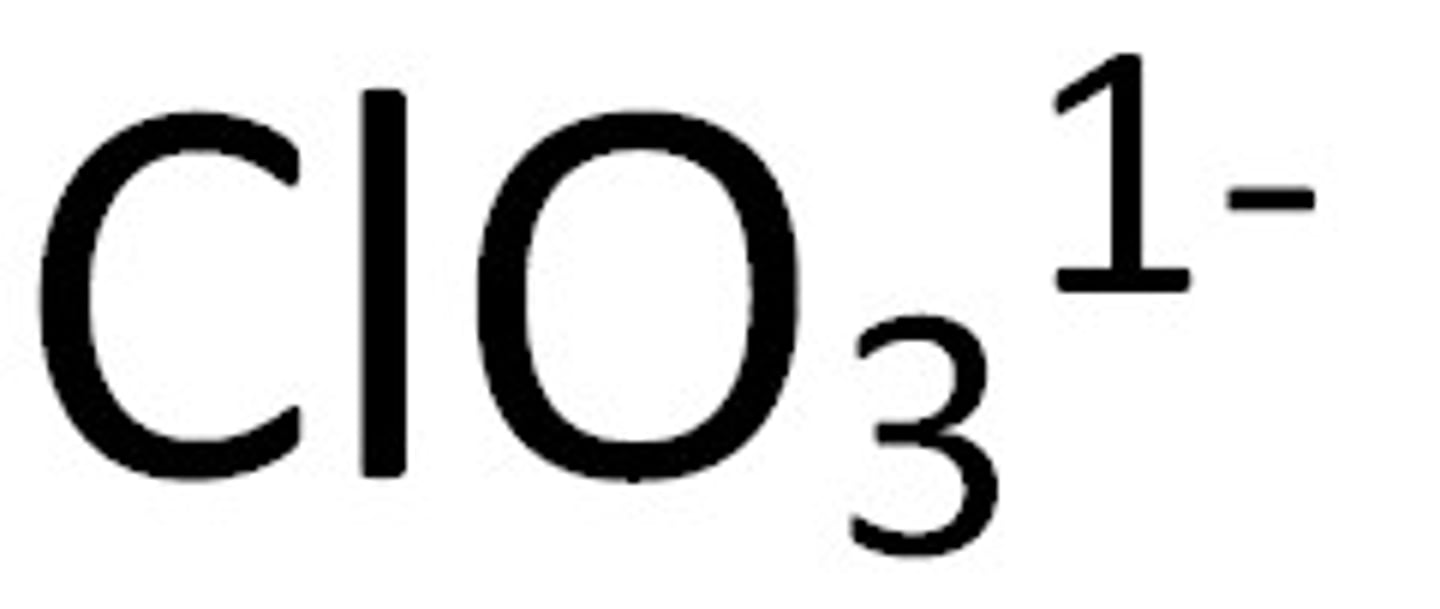
Acetates
Soluble
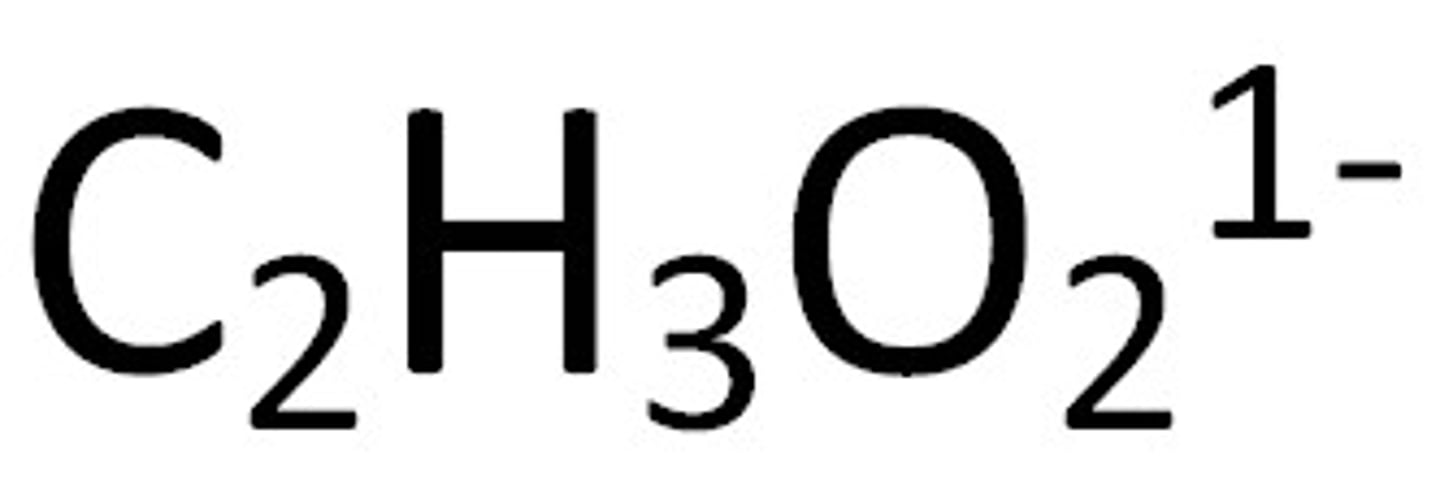
Cl-
Soluble with anything (Halides)
Br-
Soluble with anything (Halides)
I-
Soluble with anything (Halides)
Sulfates
Soluble
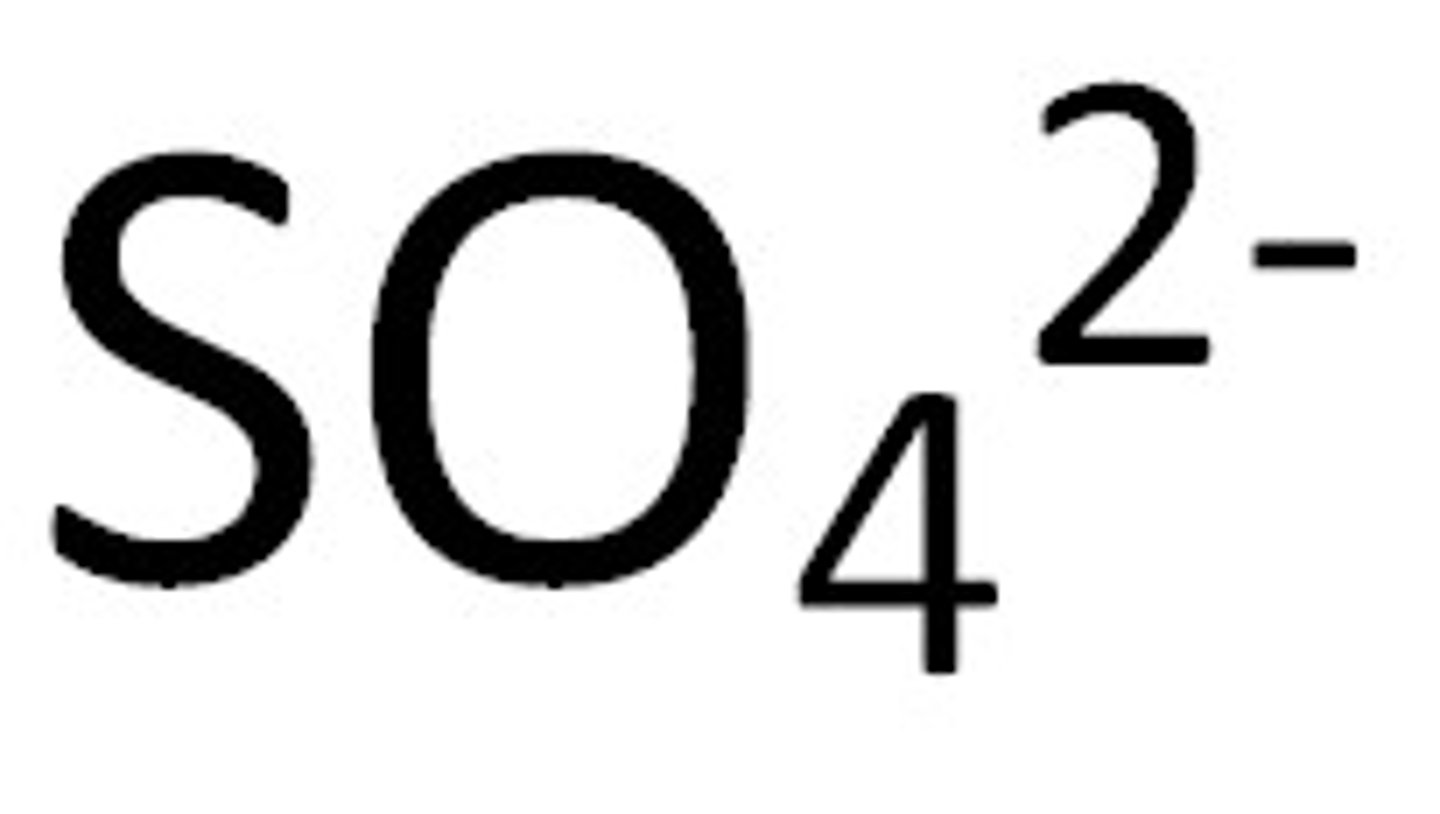
Ag+ (Halides)
Not Soluble with any Halides (AgCl= insoluble)
Pb+2 (Halides)
Not Soluble with any Halides (PbBr_2= insoluble)
Hg+2_2 (Halides)
Not Soluble with any Halides
Ag+ (Sulfates)
Not Soluble with any Sulfates
Pb+2 (Sulfates)
Not Soluble with any Sulfates
Ca+2 (Sulfates)
Not Soluble with any Sulfates
Sr+2 (sulfates)
Not Soluble with any Sulfates
Ba+2 (Sulfates)
Not Soluble with any Sulfates
Hg+2_2 (Sulfates)
Not Soluble with any Sulfates
Carbonates
Not Soluble except if it contains an alkali metal or ammonium
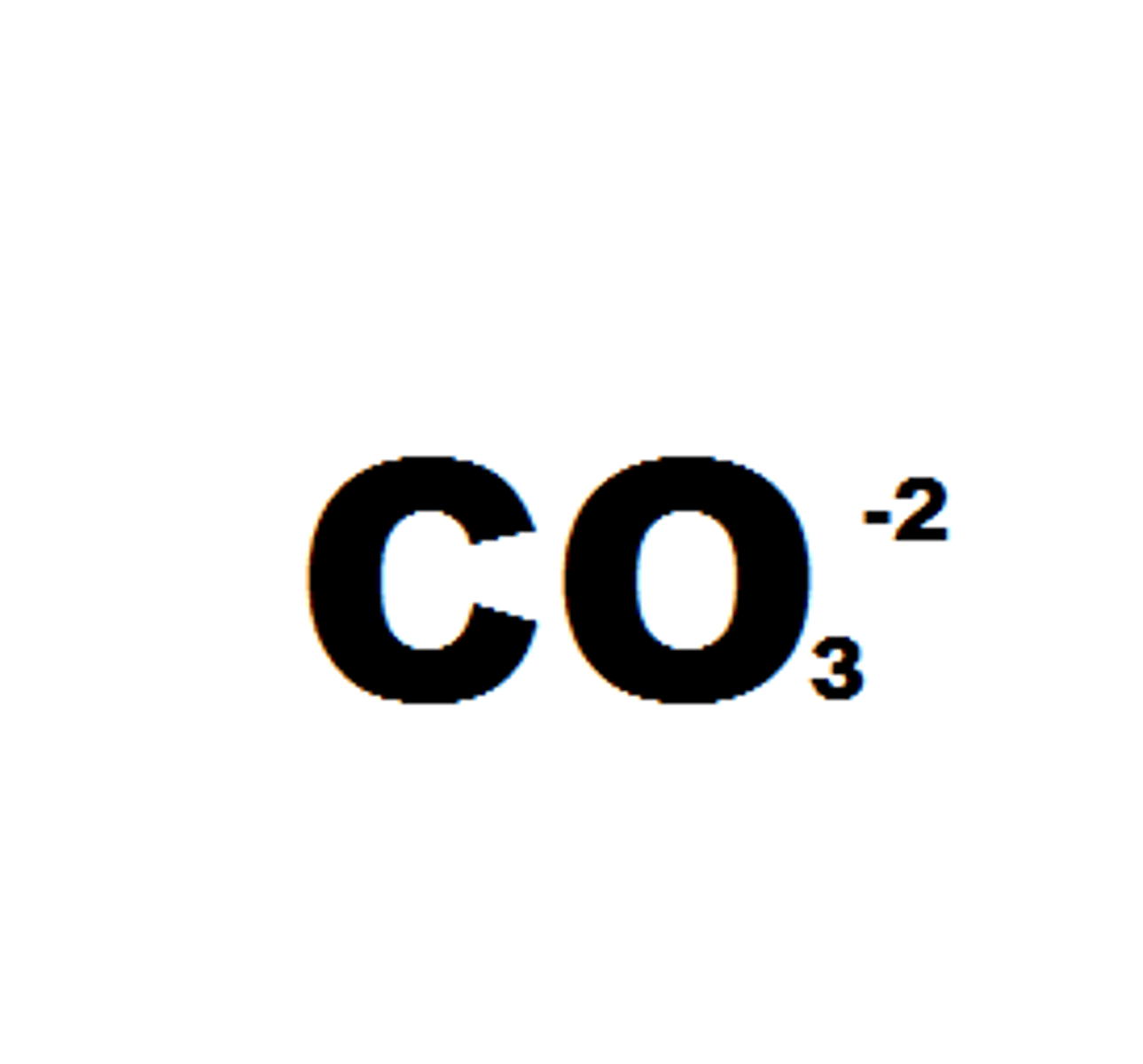
Phosphates
Not Soluble except if it contains an alkali metal or ammonium
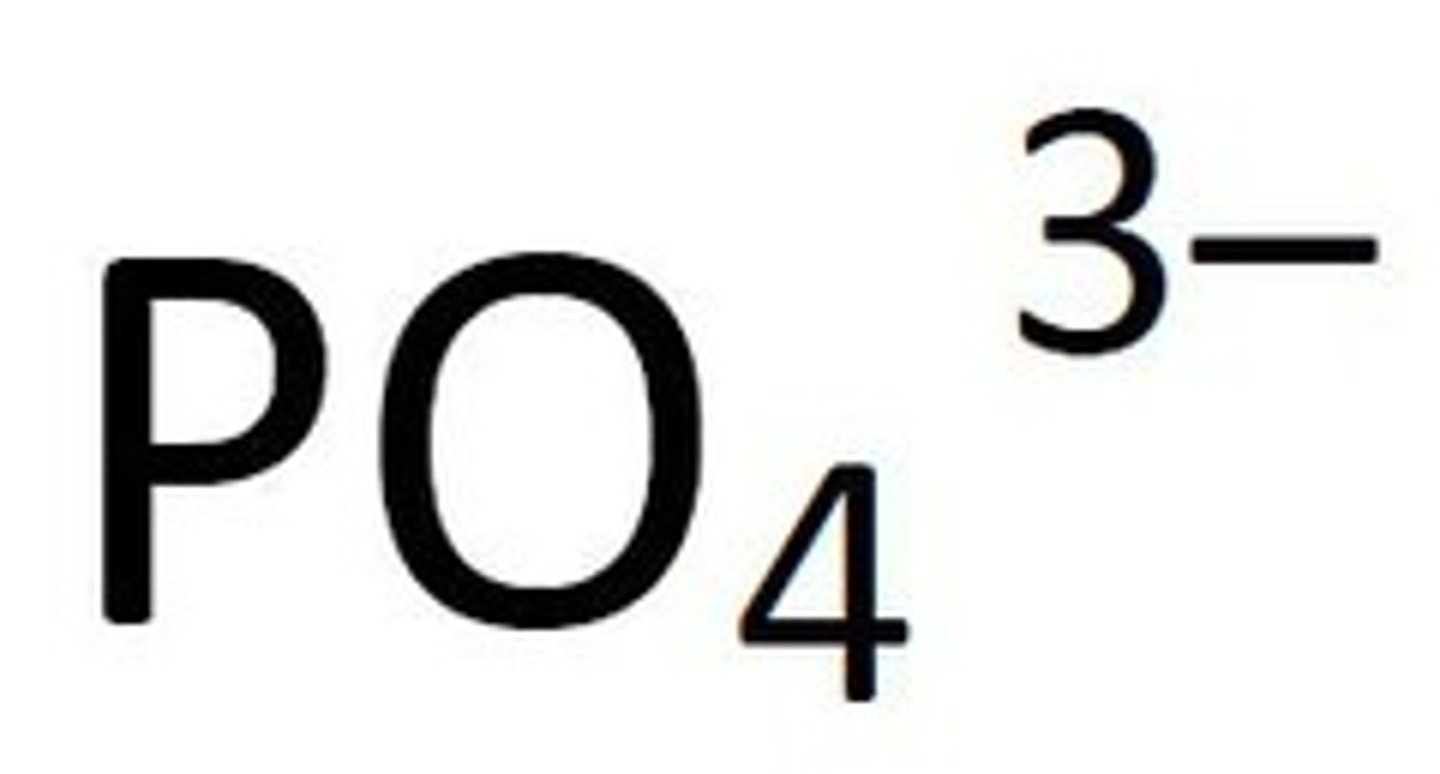
Chromates
Not Soluble except if it contains an alkali metal or ammonium
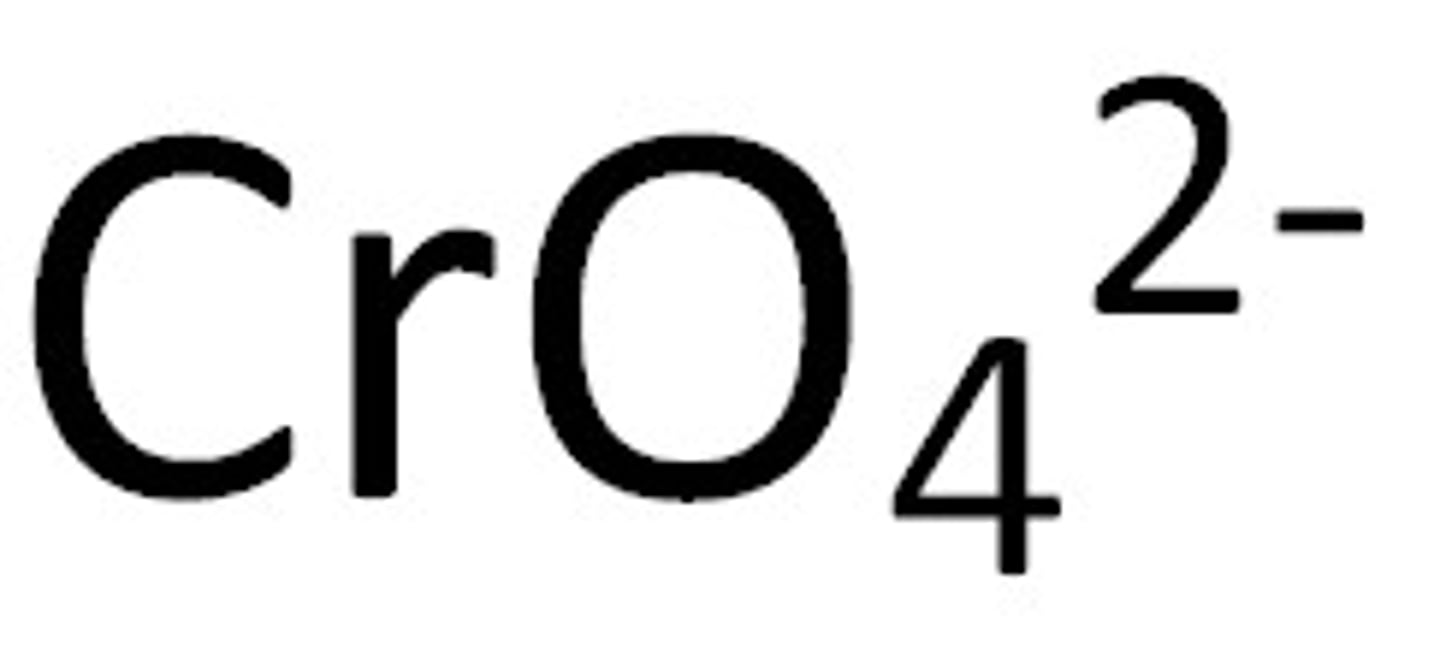
Sulfides
Not Soluble except if it contains an alkali metal or ammonium
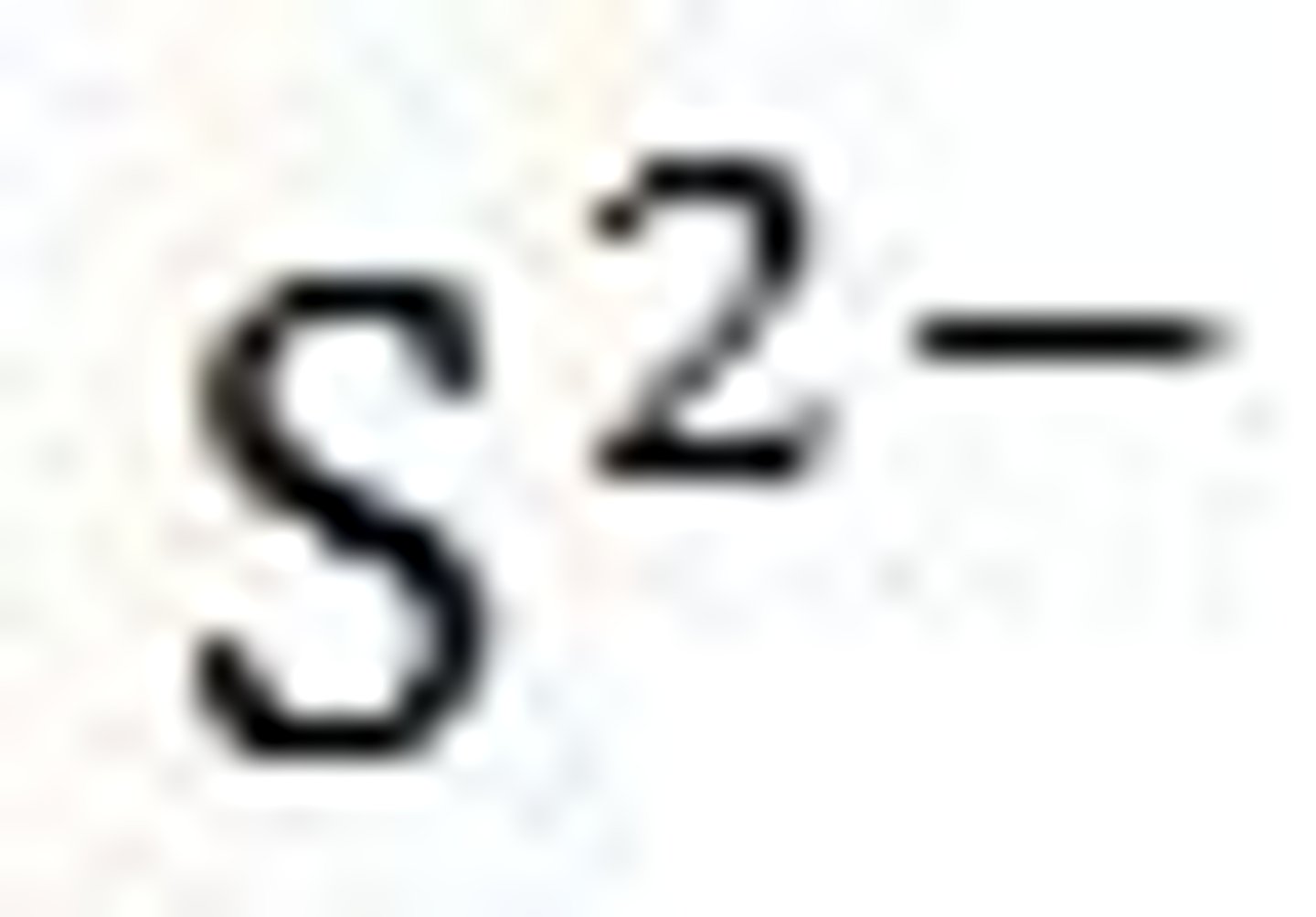
Hydroxides
Not Soluble except if it contains an alkali metal or ammonium or Ba+2