exam 1 semester 2
1/190
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
191 Terms
intermolecular forces (IMFs)
The various forces of attraction that may exist between the atoms and molecules of a substance. These forces hold multiple molecules of a substance together and determine many of a substance’s properties.
IMFs are a result of
electrostatic phenomena
IMFs are ____ forces
non-covalent
Intramolecular forces
the forces that hold atoms together in a molecule
the differences in the properties of a solid, liquid, or gas are a result of
differences in the strengths of the IMFs that make up each substance
the phase in which a substance exists depends on
the relative extents of its intermolecular forces and the kinetic energies of its molecules
intramolecular vs intermolecular forces
intramolecular are stronger
three types of IMFs
dispersion (london) forces, dipole-dipole attractions, hydrogen bonding
all IMFs are sometimes collectively referred to as
van der Waals forces
these substances have dispersion forces
all substances, polar or non polar
instantaneous dipoles are a result of
constant motion of electrons in molecules and atoms
an instantaneous dipole in one molecule or atom can then
distort the electrons in a neighboring atom or molecules producing an induced dipole
disperson forces
weak electrostatic forces from fleeting, temporary dipoles
stronger dispersion forces lead to
higher melting and boiling points
the strength of london forces increases with
increased number of electrons in a molecule, because larger molecules can be polarized more easily
surface area and dispersion forces
increasing surface area increases the strength of the dispersion forces
these molecules have dipole-dipole attractions
molecules with permanent dipoles (polar molecules)
polar molecules have
both dipoles dipole and dispersion, dipole dipole is stronger
hydrogen bonding is found in
certain polar molecules
hydrogen bonding
strong type of dipole-dipole, needs a H covalently bonded to O, N, or F
strong attraction of hydrogen bonding is accounted for by
the exposed nucleus for the lone pair of the adjacent molecule
water hydrogen bond
can make up to 4
ammonia hydrogen bond
can make up to 2
why is ice less dense than water
water molecules in their crystal form have an open-structured, six-sided arrangement. as a result, water expands upon freezing
liquids exhibit
low compressibility, lack of rigidity, and high density
three properties unique to liquids
viscosity, surface tension, capillary action
viscosity
measure of a liquid’s resistance to flow, measured by the rate in which a metal ball passes through the liquid
viscosity and IMFs
liquids with large IMFs or molecular complexity tend to be highly viscous
temperature and viscosity
viscosity of the liquid decreases
adhesive forces
force of a attraction between molecules of different chemical identities
cohesive forces
force of attraction between identical molecules
surface tension
the energy required to increase the surface of a liquid
surface tension results from
cohesive forces between molecules at the surface of a liquid
surface tension and IMFs
large IMFs have high surface tension
capillary action
flow of liquid within a porous material due to the attraction of the liquid molecules to the surface of the material and to other liquid molecules
capillary action is exhibited by
polar liquids and involves spontaneous rising of a liquid in a narrow tube
concave mensiscus
this occurs when the adhesive forces of the liquid and glass are greater than the cohesive forces of the liquid
convex mensicus
this occurs when the adhesive forces of the liquid and glass are less than the cohesive forces of the liquid
capillary action is due to
adhesive forces, based o strength of the adhesive forces, the liquid may rise or fall
extent of the rise or fall and surface tension
directly proportional
extent of the rise and fall and density of the liquid/radius of the tube
inversely proportional
thin layer chromatography
uses capillary action in which a layer of liquid is used to separate mixtures from substances
liquids are constantly
vaporizing, at a given instant some of the liquids have enough energy to escape the liquid phase
in an open container all liquid will
eventually evaporate
if a liquid is in a closed vessel with space above it
a partial pressure of the vapor state builds up in this space
when the rate at which the liquid vaporizes is equal to the rate at which the vapor condenses
a dynamic equilibrium is established
after equilibrium has been reached
the number of molecules in the gas phase does not change with time
vapor pressure
the pressure of a vapor in equilibrium with a liquid
the vapor pressure of a substance is independent
of the volume of the container
vapor pressure and temperature
vapor pressure increases with increasing temperature
boiling point
when the vapor pressure of a liquid increases enough to equal the external atmospheric pressure
boiling
as the temperature of a liquid increases, the vapor pressure increases until it reaches atmospheric pressure, temperature remains constant
normal boiling point
1 atm, 101.3 kPa, 760 torr
the lower the vapor pressure of a substance
the higher the boiling point
boiling point and atmospheric pressure
at lower pressures, the boiling point lowers
vaporization
an endothermic process
enthalpy of vaporization
the energy change associated with the vaporization process
condensation
is an exothermic process
enthalpy of condensation
the energy associated with the condensation process
enthalpy of fusion
endothermic, melting
enthalpy of freezing
exothermic, freezing
temperature remains constant
during phase change
sublimation
solid to gas state, bypassing the liquid state
deposition
gas into solid, bypassing the liquid state
sum of enthalpy of fusion and vaporization equals
enthalpy of sublimation
heating curve
a plot of temperature against time for a process where energy is added at a constant rate
heating curves depict
changes in temperature that result as the substance absorbs increasing amounts of heat
plateaus in the curve
are exhibited when the substance undergoes phase transitions
phases of a heating curve
ice, ice and water, water, water and steam, steam
heat required to increase the temperature of a substance
q = mass times specific heat times temperature change
heat absorbed or released during phase change
q = moles x change in H
triple point
the temperature at which all three phases exist simultaneously
critical point
the critical pressure and critical temperature, together, define this point
critical pressure
pressure required to produce liquefaction at critical temperature
critical temperature
the temperature above which a liquid cannot be liquefied, irrespective of pressure applied
supercritical fluid
a state which has a density characteristic of a liquid but the flow properties of a gas allowing it to diffuse through substances easily
the slope/liquid boundary is a negative slope
melting point of ice decreases with external pressure
regelation
refreezing of water derived from the melting of ice under pressure when the pressure is relieved
crystalline solids
regular arrangement, position of the components represented by a latice, the smallest repeating unit is called a unit cell
amorphous solids
disorder in structure, may undergo a transition to the crystalline state under appropriate conditions, no unit cell
types of crystalline solids
atomic solids, ionic solids, molecular solids
atomic solids
have atoms at the lattice points that describe its structure, metallic, network, and group 8A solid
ionic solids
possess ions at the points of the lattice that describe their structures
molecular solids
have discrete covalently bonded molecules at each lattice point
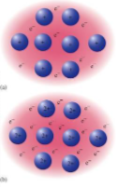
what solids are these
metallic solids, formed by metal atoms, uniform distribution of atomic nuclei within a sea of delocalized electrons, strong and nondirectional (easy to move metal atoms, hard to separate them) malleable and hard to shatter
alloy
a substance that contains a mixture of elements and has metallic properties
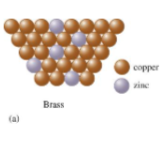
what is this
substitutional alloy, some of the host metal atoms are replaced by other metal atoms of similar size
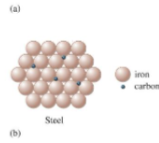
what is this
interstitial alloy, some of the holes in the closest packed metal structure are occupied by small atoms
networks solids
atomic solids that are held together by directional covalent bonds, which form solids that can be viewed as giant molecules
properties of network solids
brittle nature, hard, high melting point, ineffective conductors of heat and electricity
diamond properties
network solids, pure carbon, six membered rings, tetrahedral bonding, three-dimensional network, denser than graphite, poor electrical conductor, hard substance, good heat conductor, transparent
graphite
pure carbon, planar network solid, six membered carbon ring, each carbon atom is bonded to three others, conducts heat and electricity, strong interaction within the planes, weak interaction between planes, slippery and black
graphene
pure carbon, thinnest compound at 1 atom, exception strength due to carbon-carbon valence bond
graphene sheets can be formed into
buckballs (fullerenes), nanotubes, and stacked layers
carbon allotropes are
strong, lightweight, good conductors of electricity and heat
AM III
yellow tint, made of carbon, very hard, semi conductor, has ordered structure when viewed closely but highly disorded with less magnificatio
group 18 solids
gases, full outer shell and usually unreactive, when cooled or compressed they can become solid
ionic solids
consist of ions at the lattice points held together by electrostatic interactions, high melting points, hard, brittle, and shatter, do not conduct electricity unless they are molten or dissolved
molecular solids
neutral molecules with strong covalent bonding but weak IMFs
how to determine packing efficiency
count the number of atoms in the cell, coordination number is the number of other atoms touching the atom under consideration