Reaction profile diagrams: Energy Changes: Chemistry: GCSE (9:1)
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
Requirements for a reaction to take place
Particles must collide, with sufficient energy
Activation energy
Minimum amount of energy required to start a chemical reaction
Reaction profile diagram
A graph which shows the change in energy of a chemical reaction
Products
The new substances formed by a chemical change
Reactants
The starting material in a chemical reaction
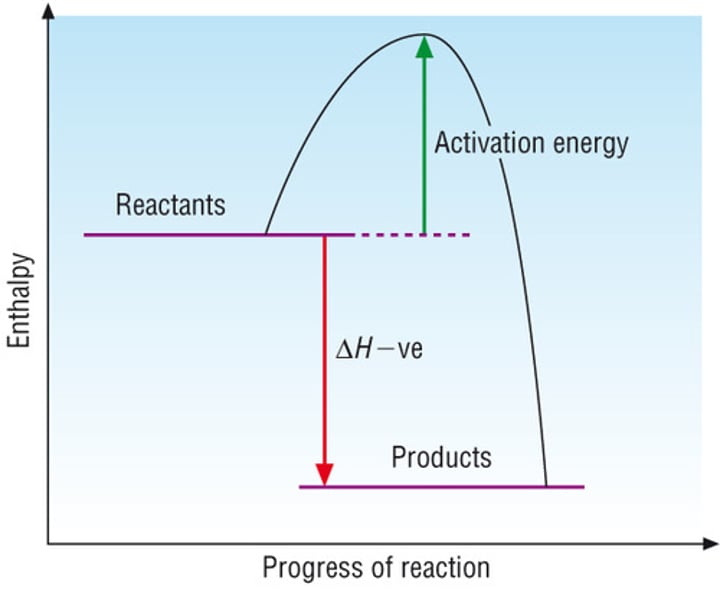
Reaction profile - exothermic
Reactants are at higher energy than products
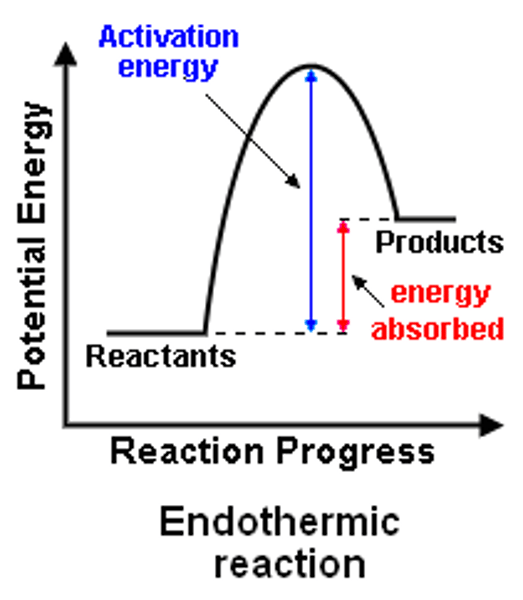
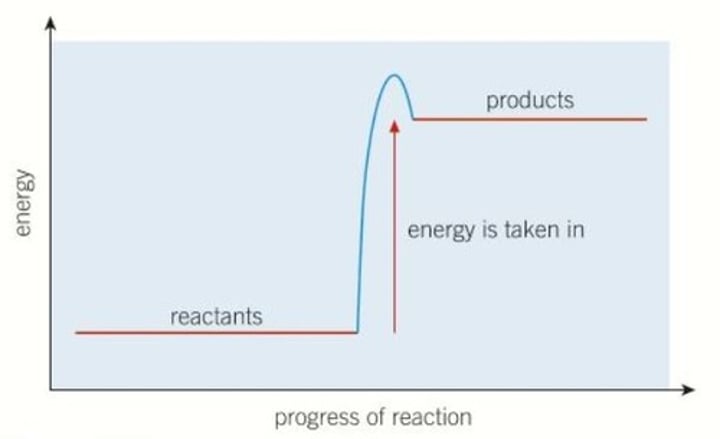
Reaction profile - endothermic
Products are at a higher energy than the reactants
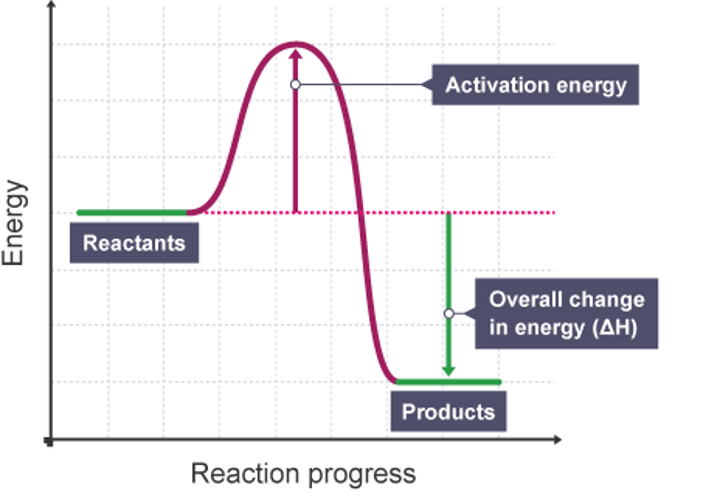
Reaction profile - activation energy
An arrow starting at reactants and going to the highest
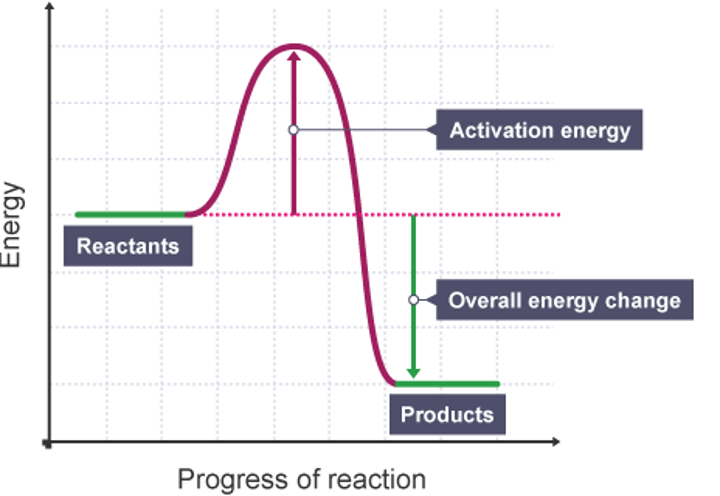
Reaction profile - overall energy changes
An arrow starting at reactions going to products
Bond breaking
Endothermic
Bond making
Exothermic
Bond energy
Average amount of energy that will break/make a bond between two atoms
Formula for overall energy change
Energy required to break bonds - energy required to break bonds
Reason for exothermic reactions
More energy is given out when new bonds are formed than is taken in to break bonds
Reason for endothermic reactions
More energy is required to break bonds than is given out when new bonds formed
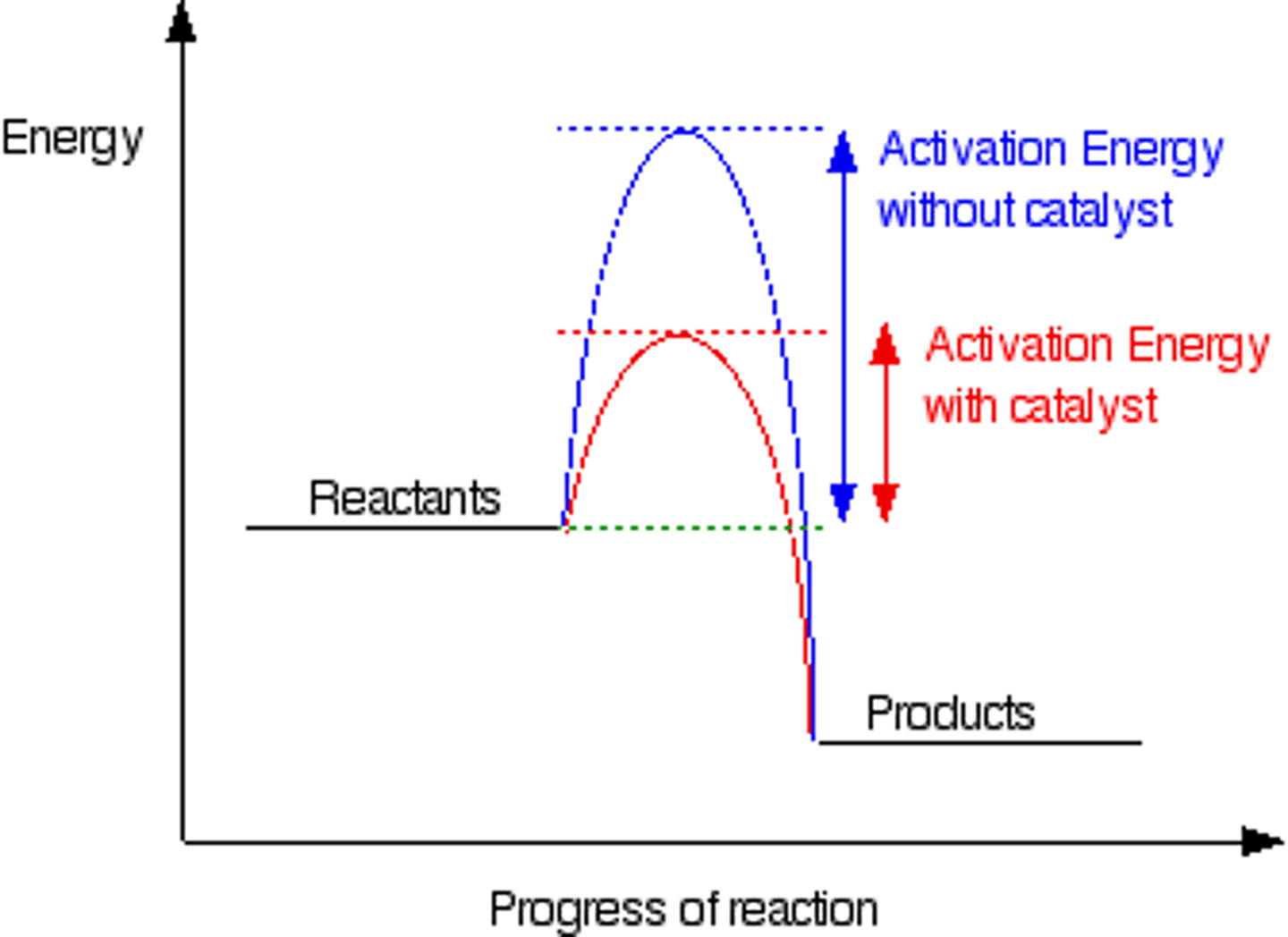
Catalyst
A substance that speeds up the rate of a chemical reaction without being used up
How catalysts work
They provide an alternate reaction pathway with lower activation energy