OChem Important Reactions
1/42
Earn XP
Description and Tags
Alkyne, Alcohol, Alkene
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
LDA (with alkyne)

LDA → Rx (alkyne)

1 equivalent of X2 (alkyne)
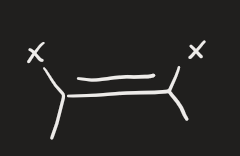
2 equivalents of X2 (alkyne)

H2O → H2SO4 (alkyne)
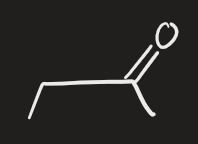
BH3 → H2O2, OH- (alkyne)

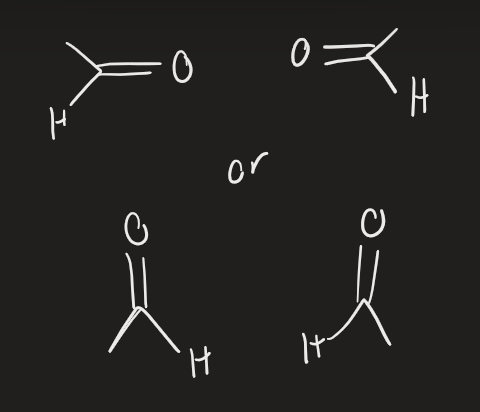
O3 → 2. (CH3)2S (alkyne)
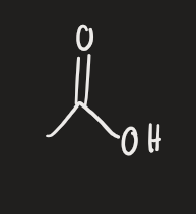
H2 → Pd (alkyne)

Na+ (cold) → NH3 (alkyne)
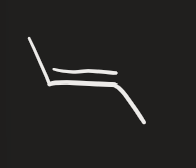
H2 → lindler’s catalyst (alkyne)
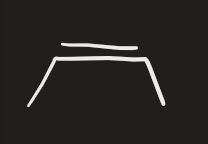
BH3 → CH3CO2H (alkyne)

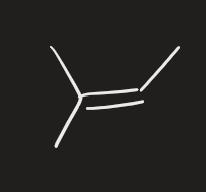
Hx (alkene)
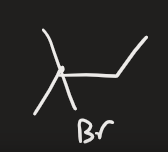

H2O → H2SO4 (alkene)
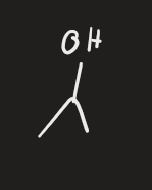

ROH → H+ (alkene)

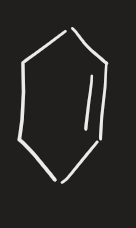
X2 (alkene)
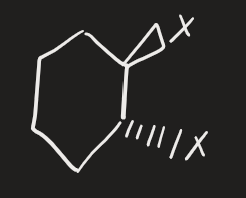
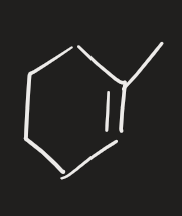
X2 → H2O (alkene)


O3 → CH3SCH3 (alkene)
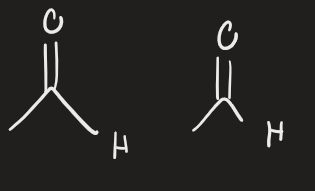
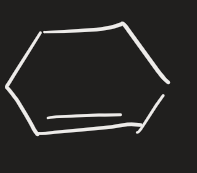
H2 → Pd (alkene)
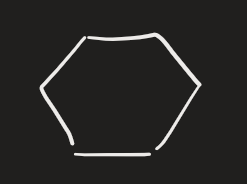
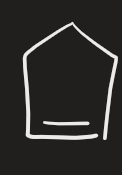
OsO4 → HSO3- (alkene)
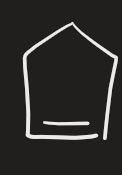
CrO3 (alkene)

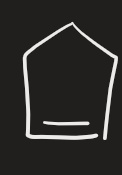
KmnO4 → OH- (cold) (alkene)
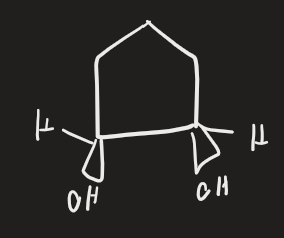

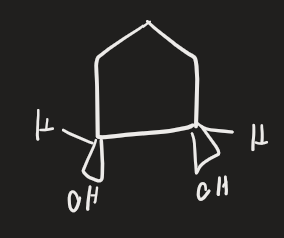
BH3 → H2O2, HO- (alkene)
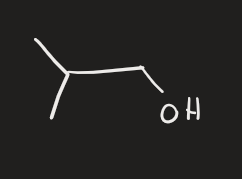
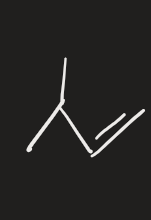
HgCl2, H2O → 2. NaBH4 (alkene)
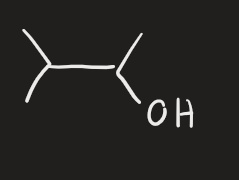
LDA → strong base (alcohol)
RO-
H2N- → strong base (alcohol)
RO-
H- → strong base (alcohol)
RO-
Li (alcohol)
RO-
Na (alcohol)
RO-
K (alcohol)
RO-
HBr (alcohol)
RBr (only for 2* or 3*)
PBr3 (alcohol)
RBr (only for 1* or 2*)
SOCl2 (alcohol)
RCl (only for 1* or 2*)
Na+ (alcohol)
-O-
TsCl → py (alcohol)
ROTs → which then reacts to the RNu-
MsCl → py (alcohol)
ROMs → which then reacts to the RNu-
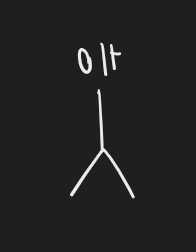
H2SO4 → heat (alcohol)


H2SO4 (alcohol)

H2CrO4 → H3O+ (1* alcohol)
carboxylic acid
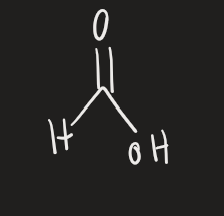
H2CrO4 → H3O+ (2* alcohol)
ketone

3* alcohol
No Reaction
PCC (1* alcohol)
aldehyde

PCC (2* alcohol)
ketone
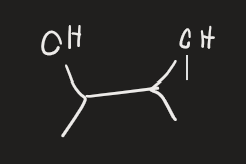
HIO4 → H2O (alcohol)