(Pharmacology of Adrenal Glands) and Corticosteroid therapy- management of side effects
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
Where are mineralocorticoids and glucocorticoids made?
Mineralocorticoids are made in the zG
Glucocorticoids are made in the zF
Androgens are made in the zR
Where are catecholamines stored and released?
Chromoffin granules which exocytose
How are steroids synthesised?
- Convert cholesterol to pregnenolone via catalysis of Desmolase
- CYP and HSDs catalyse conversion to either corticosterone to Aldosterone or 11-deoxycortisol to Cortisol
How is cortisol synthesis regulated? (The Hypothalamic-Pituitary-Adrenal (HPA) axis)
- Hypothalamus released CRH which acts on CHRH1
= CRH stimulates corticotrophin in anterior pituitary to produce ACTH
- ACTH stimulates adrenal cortex to synthesis and release cortisol
- Cortisol feeds back (negative feedback) on corticotrophin to decrease ACTH release and on hypothalamus to inhibit CRH
How is cortisol secretion controlled?
Follows a circadian rhythm
- highest in morning lowest at night
- impacted by food, stress
How does cortisol release connected to ACTH levels?
ACTH peaks with cortisol peaks
Why is pro-optiomelanocortin important?
POMC is enzymatically cleaved to make multiple peptides
ACTH comes from POMC
What is the action of ACTH on the cortical cell
- ACTH binds to receptor stimulates cAMP from ad.cycl
- PKA activation
- Lipid droplets release cholesterol into mitochondria
- In mitochondria, cholesterol is converted to Pregnenolone
- Pregnenolone leaves goes into SER, modified and back to mitochondria to convert into cortisol
- Cortisol is lipid-soluble and diffuses out to target tissues
How is cholesterol kept within lipid droplets?
Esterified into cholesterol ester trapping it
How is cholesterol released when needed to make steroid hormones?
- ACTH activated CEH enzyme which converts esterified cholesterol back to normal so it can leave droplets easily
How much cortisol is bound to CBG?
75%
How are steroid receptors activated?
-Cortisol binds causing conformational change
- HSP proteins released and block access to DNA-binding domain
- HSP moves out so receptor can bind to DNA and another can bind to ligand-bound receptor
- Transcription of target genes
How do Glucocorticoids carry out there anti inflammatory and immunosuppresive functions?
Genomic:
-Glucocorticoids are small, lipophilic molecules – readily pass through cell membrane and into the cytoplasm
-Bind to glucocorticoid receptors which results in shedding of heat shock proteins and formation of a complex which then translocates to the nucleus
- The complex binds to specific DNA sites resulting in stimulation or suppression of gene transcription
- Results in suppression of synthesis of pro-inflammatory cytokines and stimulation or activation of anti-inflammatory cytokines
OR
Non-genomic:
- At high doses, glucocorticoids bind to glucocorticoid receptor on T – lymphocytes resulting in impaired receptor signalling and functioning of T cells in their immune response
What are corticosteroids?
Any group of steroid hormones produced by the adrenal cortex or made synthetically
What should we be aware of when using using corticosteroids considering how it affects immune response?
- The multiple anti-inflammatory and immunosuppressive properties of corticosteroids
can predispose patients to infections: activation of latent infection of exacerbation of
intercurrent infection
- There may be altered clinical presentation of infections and severity of infections may
be masked due to immunosuppressant activity of corticosteroids
- Particular concern around TB and Varicella history
What is the most common cause of adrenal insufficiency?
chronic administration of high dose corticosteroids
What is the main role of corticosteroids and and how does can Adrenal insufficiency occur linked to them?
- Corticosteroids suppress the hypothalamic-pituitary-adrenal axis = adrenal glands
lose the ability to produce cortisol
- AI occurs when corticosteroids are withdrawn too rapidly or in stressful conditions
(surgery/infection); higher levels of corticosteroids may be required
How can we avoid adrenal insuffiency ?
-Avoid abrupt withdrawal, especially with prolonged courses
-Increase corticosteroid dose in illness/trauma/surgical procedure (diminished adrenocortical response)
-Patient counselling and steroid treatment card

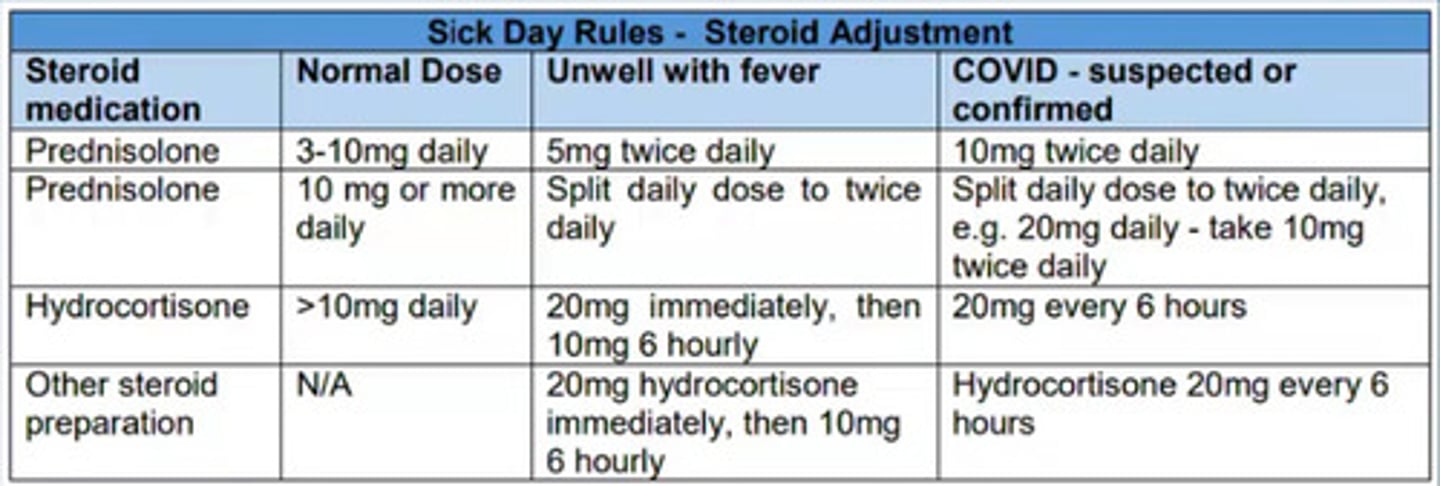
Sick day rules - Steroid adjustments
Explains what dose patients on corticosteroids should be taking when they are suffering with certain conditions e.g fever/COVID
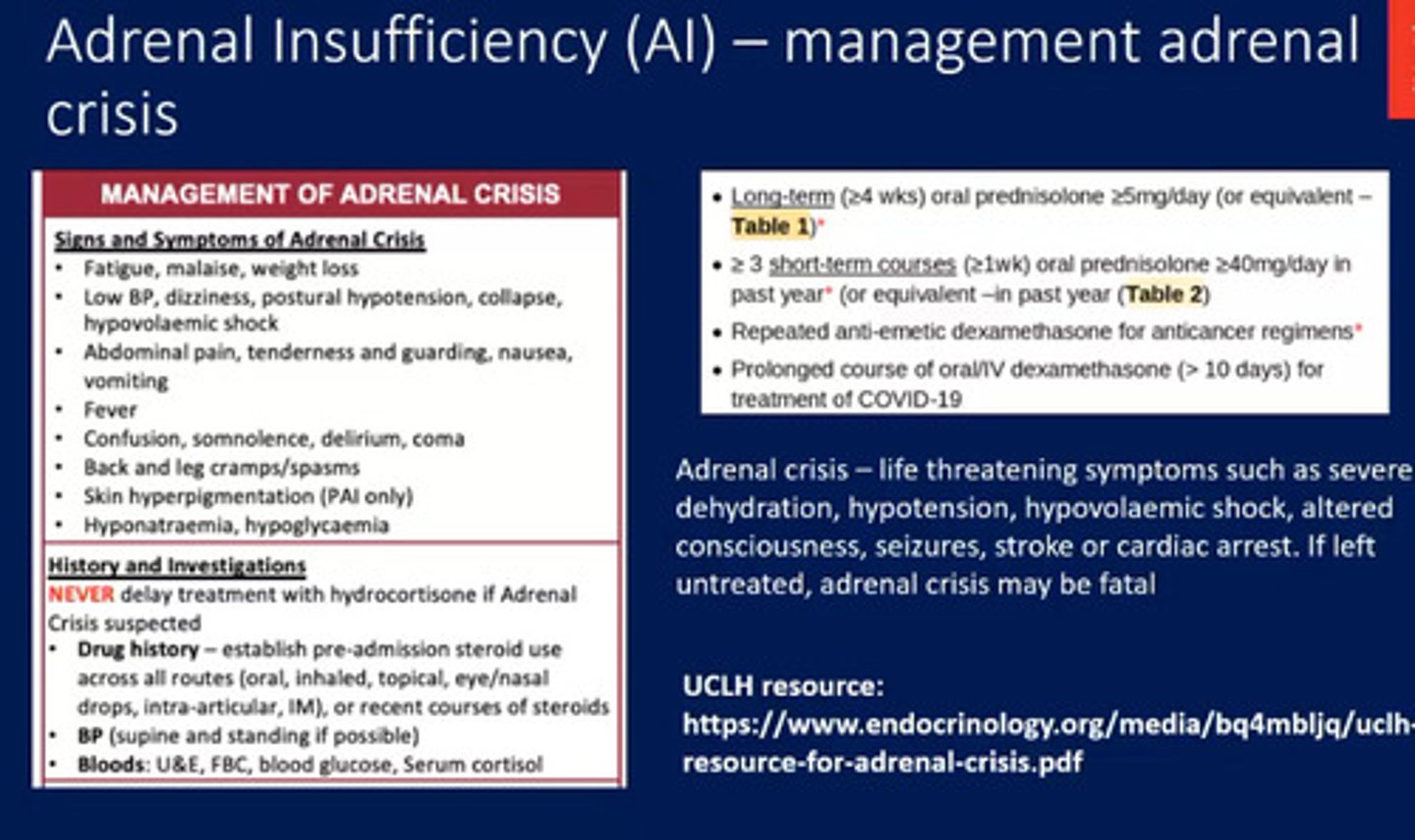
Signs and symptoms of Adrenal crisis?
Monitoring?
-Patients in the long term section we would be concerned about for adrenal insufficiency
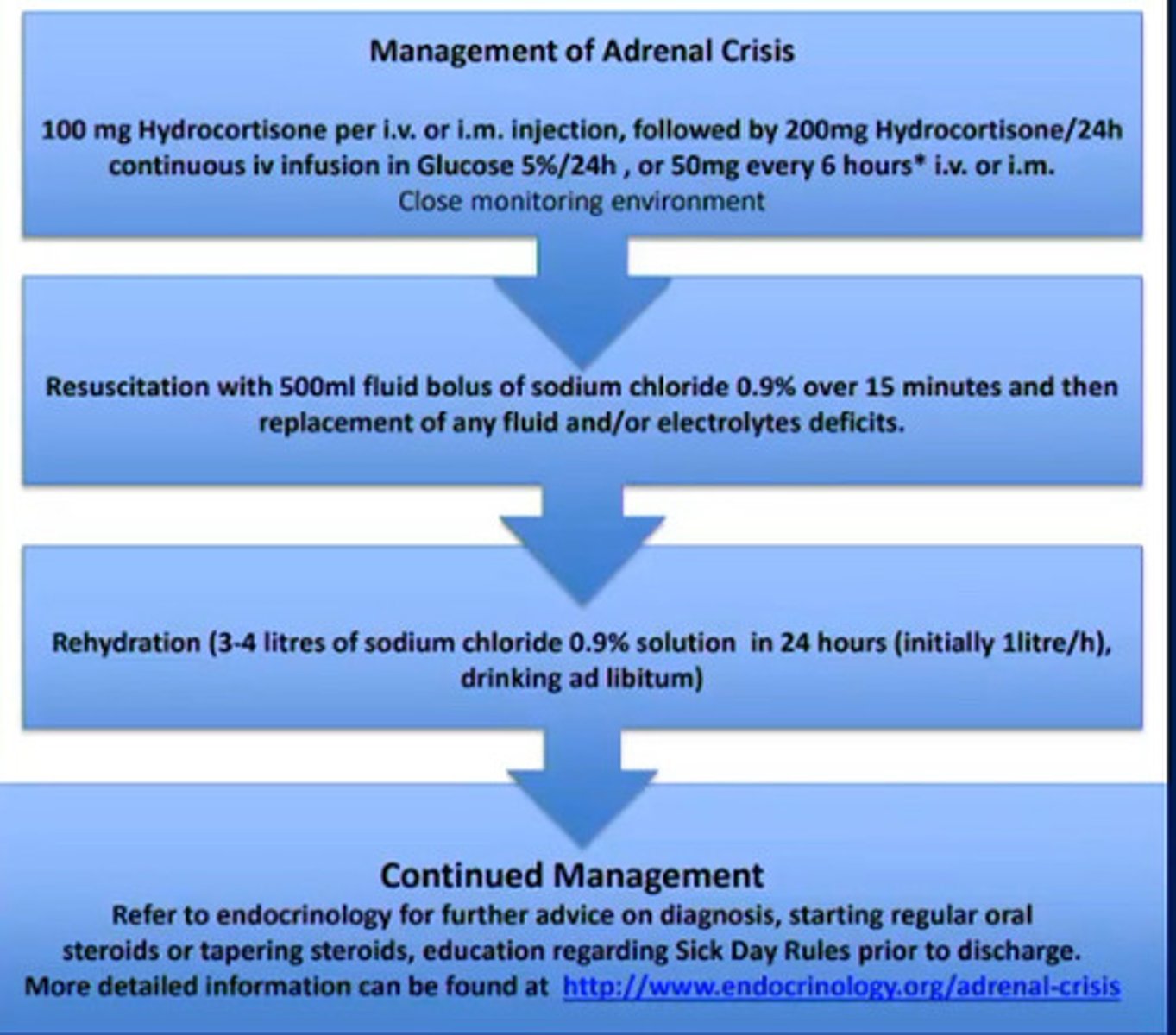
How Do we manage an adrenal crisis (adrenal insufficiency)?
Why do we prescribe steroids? - Glucocorticoids
1) Replacement therapy e.g. adrenal insufficiency such as Addison's Disease OR Congential adrenal hyperplasia (CAH) – genetic condition – large adrenal gland, lacking enzyme proteins required to make cortisol or aldosterone
2) Systemic symptomatic treatment:
-Prophylaxis e.g. renal transplant, fetal lung maturation
-Acute e.g. asthma, copd, acute exacerbation of autoimmune diseases
-Long term e.g. chronic inflammatory diseases like IBD
Why do we prescribe steroids? -mineralocorticoids
-Heavily involved in regulation of water and electrolyte balance
-Use in practice limited to replacement therapy in adrenal insufficiency (replacement of aldosterone)
What should we consider when thinking about the SE's of corticosteroids ?
-Heavily involved in regulation of water and electrolyte balance
-Use in practice limited to replacement therapy in adrenal insufficiency (replacement of aldosterone)
What should we remember linked to live vaccines and patients on a high dose of Corticosteroids?
- Do not give live vaccines to patients on high CS due to immuno-suppression
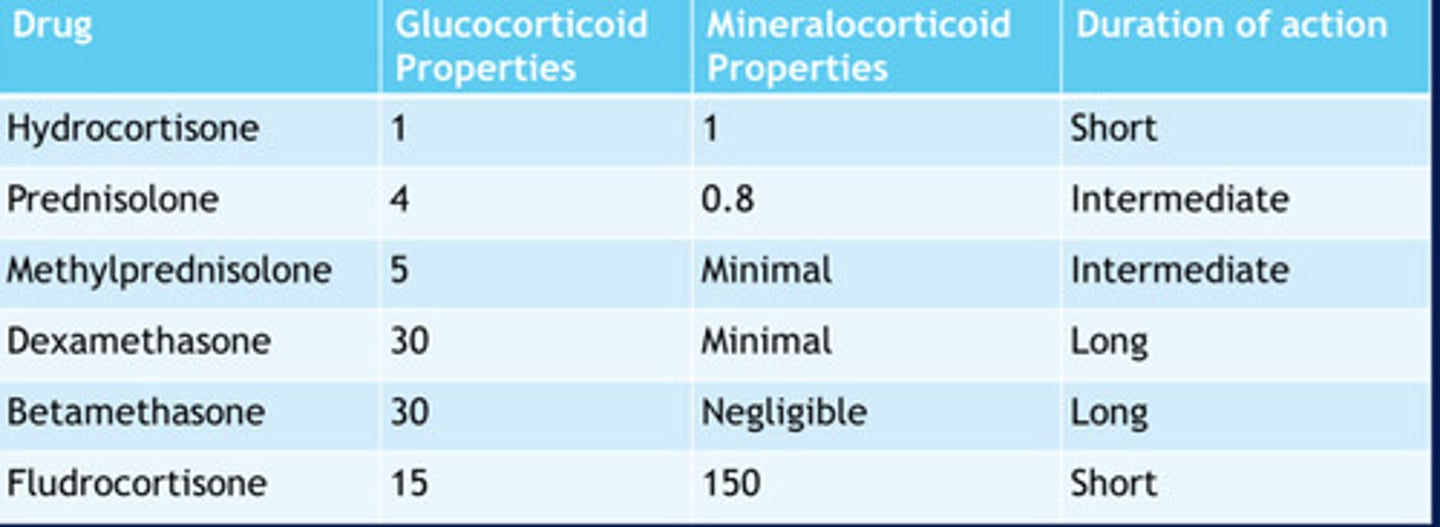
Corticosteroid drug chart
What are the metabolic effects of chronically elevated glucocorticoids?
- Less glucose uptake
- Increase in gluconeogenesis
- Hyperglycaemia
- Decrease in protein synthesis
- Increase in protein breakdown
- Muscle wasting
- Decreased calcium gut absorption
- Increased osteoclasts decreased osteoblasts = bone depletion
- Ant-inflammatory effects
- Reduced wound healing, repair
- Decreased COX-2, cytokines, blood complements, histamine, IgG
How do mineralocorticoids (MCs) regulate water retention and electrolyte balance?
Increase Na and K
Does cortisol have higher affinity for MCs or GCs (Glucocorticoids)?
MC
What are the two forms in which glucocorticoids exist?
Active form as cortisol
Inactive form as cortisone
How and where is cortisol transformed from active to inactive and vv?
- D2 isoform is expressed in aldosterone and converts cortisol to cortisone
- D1 isoform expressed in liver, adipose and muscle and converts cortisone to cortisol
What is Cushing's Syndrome - how does corticosteroids cause it ?
-irregularity of fat
tissue distribution
- Corticosteroids have effects on metabolism of fat tissue causing irregularity of fat
tissue distribution
What is the predominant cause of Cushing's?
Pituitary adenoma causing excess ACTH
What is Cushing's caused by?
- Prolonged exposure of exogenous GC drugs
- Osteoporosis
- Obesity
- Pituitary tumour producing excess ACTH
What are ACTH-independent causes of Cushing's?
Adrenal adenoma, hyperplasia, carcinoma
What are the clinical signs of Cushing's?
Moon face/buffalo hump
Irregular fat tissue distribution
Obesity
Hypertension
Hirsutism
Striae
Acne
Brusing
Menstrual disorders
Impotence
Glucose intoleracne
Diabetes
What are the drug treatment options for Cushing's?
- Inhibit steroid synthesis with Metyrapone or Ketoconazone
- Inhibit ACTH with Pasireotide, Cabergoline
- Inhibit GC = Mifeprestone
As we know Corticosteroids can be linked to Cushing's - -irregularity of fat
tissue distribution - How might this effect Blood pressure and lipid levels in patients?
-increasing cardiovascular risk due to weight gain and high levels of cholesterol and triglycerides
-Affinity for the mineralocorticoid receptor leads to sodium retention, fluid retention and therefore increased blood pressure, further contributing to cardiovascular risk
What is the effect of Hyperglycaemia and Diabetes in patients on corticosteroids?
Who is at risk of this?
- 1) Increase glucogenesis = increase in blood glucose (- Effects on blood glucose seen 4-8 hrs post-dose)
- 2) Increase in insulin resistance and reduce glucose uptake in muscle, adipose
tissue and the liver
Who is at risk of this?
- Risk factors: pre-existing diabetes, obesity, family hx, previous gestational diabetes,
PCOS
Counselling for Endocrine, metabolic and cardiovascular side effect management
- calorie controlled diet may minimise weight gain
- monitor blood glucose and if necessary advise on diet modification/start hypoglycaemic agents
- monitor triglycerides, low saturated fat diet/start statin
- Low salt diet reduces sodium and water retention
- Diet rich in potassium (fish, fruit, veg) to counteract potassium loss. Potassium supplementation if necessary - Blood pressure monitoring
How do Corticosteroid patients affect patients' bones?'
Effects?
Risk factors?
- Can cause Musculoskeletal Osteoporosis, Bones become brittle + fragile due to loss of bone tissue = increased fracture risk
Effects of the Corticosteroids?
-reduced osteoblasts = reduced bone formation
-increased bone resorption
due to increased osteoclast activity
-reduced absorption of calcium and phosphate
Risk factors: -dose and duration of corticosteroids -age -body weight -underlying disease (RA, PMR, IBD, transplant) -smoking, alcohol XS
There is also a risk of Osteonecrosis:
- Death of bone tissue due to lack of blood supply
- Pathogenesis of corticosteroid relates osteonecrosis not fully known:
o Increased fat in marrow = increased pressure and compression of blood
vessels = ischaemia
o Increased lipids cause fat emboli occluding blood vessels
- Symptoms: joint pain and stiffness, femoral head most common affected joint but can
be any large joint
What are the causes of Addison's?
Primary = adrenal cortex destruction
Secondary = lack of ACTH
What are the clinical features of Addison's?
Weakness
Fatigue
Anorexia
Weight loss
Hypotension
Hyperpigmentation
Gi disturbance
Salt craving
What is the treatment of Addison's?
Replacement therapy with Fludrocortisone
How do corticosteroids cause adrenal insufficiency?
Suppress the hypothalamic-pit-adrenal axis
- withdrawing too quick
- stress conditions e.g., infection, surgery
How do chronic corticosteroids impact the immune response?
Immunosuppression can cause activation of latent infection or exacerbation of current infection
What is the effect of hyperglycaemia?
- Increased gluconeogenesis
- Increased insulin resistance and reduce glucose uptake in muscle, adipose and liver
How can corticosteroids cause osteonecrosis?
Death of bone due to lack of food supply
- increased fat in marrow - increased compression of blood vessels = ischaemia
- increased lipids creating fat emboli occluding vessels
- joint pain/stiffness
How does corticosteroids cause myopathy, and what is it?
How can we test this in a patient?
-Myopathy is the Reduction in muscle protein synthesis and protein catabolism
Hypokalaemia causing muscle weakness as a SE
- Patients demonstrate by standing from sitting without using hands
How can we prevent Osteoporosis?
Management of Musculoskeletal SE's of Corticosteroids?
Osteoporosis prevention:
-- undertake weight bearing activity, stop smoking, avoid excess alcohol
- consider supplements to prevent osteoporosis
-DEXA scans to monitor bone mineral density
- consider hormone replacement therapy in those most at risk of osteoporosis
Management:
-Reduction/withdrawal of steroids if possible e.g. myopathy
- Surgery and/or joint replacement sometimes necessary with osteonecrosis
- Analgesia
What are the psychiatric disturbances associated with high-dose corticosteroids and why?
How do we manage Psychiatric + cognitive disturbances?
-thought to be due to abnormalities in the HPA axis - alteration in secretion of cortisol, imbalance of mineralocorticoid and glucocorticoid receptor stimulation - range of psychiatric and cognitive symptoms
Symptoms:
Insomnia
Mood change
Depression
Psychosis
- usually symptoms subside with stoppage
- can occur w/ withdrawal of corticosteroids
How do we manage Psychiatric + cognitive disturbances?
-Consider patient history - risk vs benefit of steroids
-Tapering down the dose
-To ensure there is no disturbance in the patients sleeping patient should be counselled to take the corticosteroid as a single dose in the morning.
How do corticosteroids affect the GI?
GI side effects:
Increase acid secretion, reduce mucus, delay ulcer healing
- causes peptic ulcers, GI bleeds, pancreatitis
How do we manage GI SE's?
-Patient counselling to report symptoms
-GI side effects minimised by administration of corticosteroid with food
-Consider prophylactic agent for those who have risk factors of peptic ulcer disease (not all patients will need a PPI)
-Think about other medication the patient is on that may increase risks of GI side effects e.g. NSAIDs, SSRIs
What are the ocular effects of corticosteroids?
management?
Glaucoma - due to raised intraocular pressure associated with glucocorticoids
Cataracts, Blurred vision - -thought to be due to glucocorticoid binding with lens proteins resulting in destabilisation
Secondary fungal/viral eye infection
Optic nerve damage
Mangement – regular checks, dose reduction/discontinuation, medical management of these conditions
What are the skin effects of corticosteroids?
Management
Atrophy/fragility (thinning of skin) - glucocorticoids suppress cutaneous cell proliferation, decreased synthesis of epidermal lipids
Purpura (blood spots/skin haemorrhage) may occur due to severe atrophy
Delayed wound healing - glucocorticoids suppress the early inflammatory phase which is essential for would healing, and impact on growth factors/proteins required for would healing
Management: – counselling is key e.g. thinly apply steroid creams, not applying to broken skin, consideration of wound healing around surgeries and whether doses can be optimised around surgery (adrenal crisis vs wound healing
How would you manage the various corticosteroid side effects?
- Carry a steroid card
- Avoid abrupt stoppage
- Avoid in infection
- Diet change/monitor blood glucose/tri
- Weight-bearing activity encouraged
- Stop smoking/excess alcohol
- DEXA bone scans
- HRT in osteoporosis risk
- Patient counselling
- Low salt diet, potassium-rich diet, BP monitoring
- Administer with food
- Avoid PPIs where possible
- TB and varicella history
- Lowest dose, shortest time
- Treat pre-existing conditions before initiating treatment
How would you manage the various corticosteroid side effects? EXTENDED
- Carrying a steroid card: advises not to stop abruptly to avoid adrenal suppression
- Adrenal suppression: avoid abrupt withdrawal and increase corticosteroid dose in
illness/trauma/surgical procedure (diminished adrenocortical response)
- Immune response: patient counselling/symptom recognition = avoid contain with
certain groups
- Increased susceptibility: chicken pox, measles
- Endocrine and metabolic: warning of SEs and calorie-controlled diet may minimise
weight gain, monitor blood glucose and if necessary advise diet to change/ start
hypoglycaemic agents, monitor triglycerides, low saturated fat diet/start statin
- Musculoskeletal: counsel to undertake weight bearing activity, stop smoking, avoid
excess alcohol, consider supplements to suppress osteoporosis, DEXA scans to
monitor bone mineral density, consider hormone replacement therapy in those most
as risk of osteoporosis
- Behavioural changes: mood swings, euphoria, depression-symptoms respond to
tapering dose
- Sleep disturbance- patient counselling to take corticosteroid as a single morning
dose
- Fluid and electrolyte balance: low salt diet reduced sodium and water retention,
potassium-rich diet to counteract potassium loss, BP monitoring
- GI disease: patient counselling to report symptoms, minimised by administration of
corticosteroid with food, consider prophylactic agent for those with peptic ulcer risk
factors
How to appropriately manage patients on corticosteroids to reduce harm?
• Ensure we have an appropriate history for the patient prior to treatment
• To minimise corticosteroid-induced side effects, the lowests effective dose should be used for the minimum period of time possible
• Any pre-existing co-morbidities that may increase the risk of corticosteroid induced side effects should be treated/optimised prior to corticosteroid initiation
• Patient consultation/counselling