11. SNAREs 1: Membrane fusion machinery
1/45
Earn XP
Description and Tags
complete
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
What is membrane fusion?
Process where two membranes merge, allowing the contents of one compartment to mix with another.
Name a key example of membrane fusion in the nervous system.
Synaptic vesicle fusion, which facilitates communication between neurons and muscles.
How does membrane fusion play a role in the pancreas?
Secretory granule fusion occurs in both endocrine and exocrine functions of the pancreas, releasing hormones and digestive enzymes.
Which proteins are secreted by membrane fusion in hepatocytes and plasma cells?
Hepatocytes secrete albumin, and plasma cells secrete antibodies through membrane fusion.
What role does membrane fusion play in mucus secretion?
Mucus is secreted by epithelial mucosal cells via membrane fusion.
Which technique can be used to visualize secretory vesicles?
Electron microscopy.
What are the three main approaches used to identify the machinery of vesicle transport?
1) Biochemical reconstitution
2) Yeast genetics
3) Cloning
What is biochemical reconstitution used for in vesicle transport studies?
Used to recreate vesicle transport systems in vitro, enabling the discovery and purification of the proteins and enzymes involved based on their functions.
What is the intra-Golgi transport assay?
A cell-free system used to study transport between Golgi compartments, which helps identify the proteins responsible for vesicle transport.
In the intra-Golgi transport assay, what is the role of the "donor" Golgi-containing fraction?
The "donor" Golgi-containing fraction comes from VSV-infected 15B mutant cells and is used to provide vesicles for transport in the assay.
What is the role of the "acceptor" Golgi-containing fraction in the intra-Golgi transport assay?
The "acceptor" Golgi-containing fraction comes from uninfected wild-type cells and is used to receive vesicles in the assay.
What was found in the "acceptor" Golgi-containing fraction that proved vesicle transport had occurred?
The presence of transferred viral glycoproteins from the "donor" Golgi in the "acceptor" fraction proved that vesicle-mediated transport had successfully occurred.
What is NSF and how was it identified?
N-ethylmaleimide Sensitive Factor, is an ATPase essential for vesicle fusion.
NSF was identified when the reagent N-ethylmaleimide inhibited vesicle fusion, making NSF necessary to overcome this block.
What happens to NSF when membranes are salt-washed?
NSF can no longer bind to them, indicating the need for an additional factor for its attachment.
What is SNAP, and how was it identified?
Soluble NSF Attachment Protein, was identified as the factor required for NSF to bind to membranes after they were salt-washed.
How do SNAPs bind to membranes, and what proteins are involved?
Via hypothetical proteins called SNAP REceptors (SNAREs), which mediate the attachment of NSF to the membrane for vesicle fusion.
How does NSF interact with membranes, and what is its cycling mechanism?
NSF cycles on and off membranes in an ATP-dependent manner.
It binds to membranes when ATP is present, facilitating the disassembly of SNARE complexes.
Once its function is complete, NSF releases from the membrane, allowing for the recycling of SNARE proteins for subsequent vesicle fusion events.
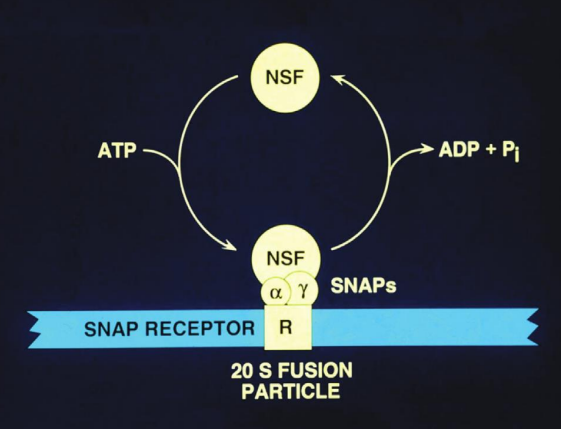
What are Sec mutants, and what is their significance in studying vesicle transport?
Temperature-sensitive mutants in yeast that are crucial for studying the vesicle transport machinery. They are involved in various stages of secretion
The study of these mutants has demonstrated that the vesicle transport mechanisms are conserved between yeast and mammals.
E.g SEC1, SEC17 & SEC18
What do each of these Sec mutants encode?
SEC1
SEC17
SEC18
SEC1 (SNARE binding protein), SEC17 encodes a-SNAP, SEC18 encodes NSF.
All are involved in SNARE function.
How were synaptic vesicle proteins VAMP and Syntaxin cloned?
Antibodies were raised against synaptic vesicles purified from electric rays. The antibodies were then used to expression clone VAMP and Syntaxin.
What are VAMP and Syntaxin crucial for?
Synaptic transmission.
What is the effect of clostridial neurotoxins, such as tetanus and botulinum B, on VAMP?
Clostridial neurotoxins, specifically tetanus toxin and botulinum toxin B, cleave VAMP (synaptobrevin), which is essential for synaptic vesicle fusion.
What could the treatment of synaptic vesicle proteins (SSV) with tetanus toxin demonstrate?
Analysed using SDS-PAGE gel, revealing the cleavage of VAMP and demonstrating the toxin's impact on neurotransmitter release.
What was discovered during the biochemical purification of SNAREs, specifically regarding SNAP25?
identified SNAP25 (synaptosomal-associated protein of 25 kD) as part of a large protein complex that disassembles upon ATP hydrolysis.
This finding highlights the role of ATP in regulating SNARE complex dynamics, which is essential for vesicle fusion and neurotransmitter release.
What are the key points of Rothman's SNARE hypothesis? (4)
1) SNAREs for each transport step within the cell.
2) SNAREs should provide specificity to vesicle transport.
3) SNAREs should be sufficient to drive lipid bilayer fusion.
4) Proposed that NSF and ATP hydrolysis catalyses membrane fusion (this bit is wrong!)
How many SNAREs are encoded in the human genome, and what is their role?
38
Involved in various transport steps and fusion reactions within cells. These proteins play critical roles in mediating the specificity and efficiency of vesicle trafficking and membrane fusion processes.
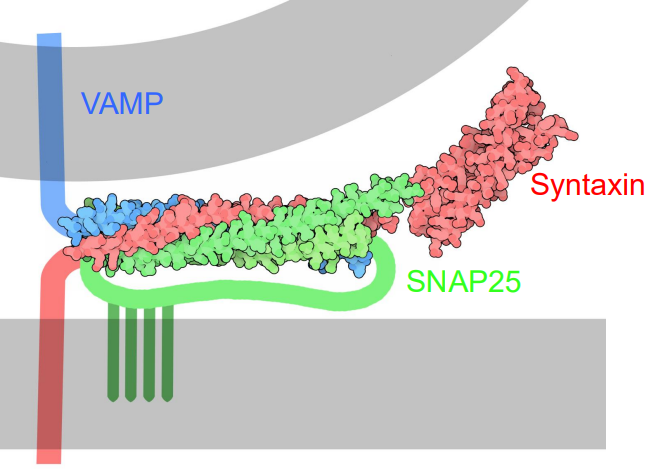
What is the crystal structure of the neuronal SNARE complex?
A bundle of, highly twisted α helices formed by the SNARE motifs of VAMP, syntaxin, and SNAP-25.
The SNARE proteins zipper together in a parallel coiled coil formation.
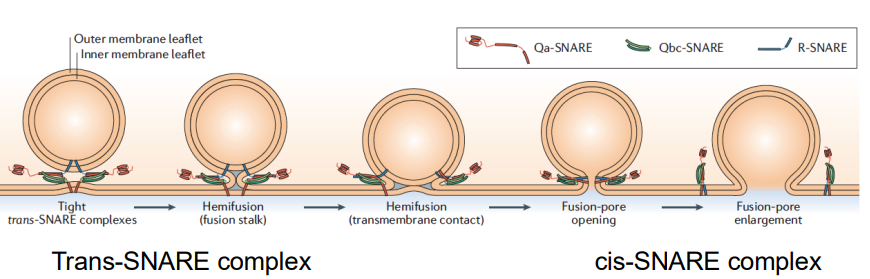
How does the crystal structure of the SNARE complex explain SNARE function and membrane fusion (hint: cis/trans)?
SNARE zippering generates energy to drive membrane fusion.
The formation of the trans-SNARE complex (between different membranes) and the cis-SNARE complex (within a single membrane) helps bring two membranes together, overcoming the energetically unfavourable process of fusion.
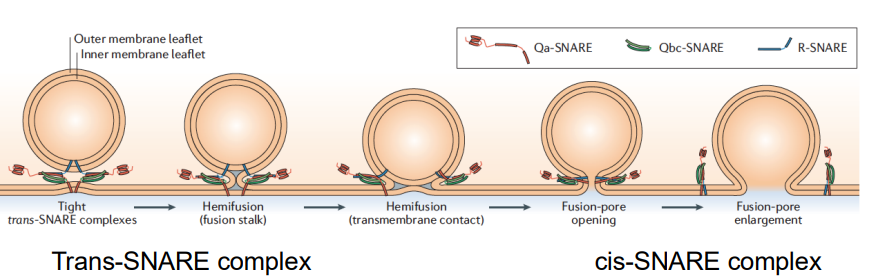
How do the trans-SNARE and cis-SNARE complexes work to bring membranes together during fusion?
The trans-SNARE complex forms when SNARE proteins from two opposing membranes (the vesicle and the target membrane) interact, effectively tethering the two membranes together.
Once they are close, the cis-SNARE complex forms as the SNARE proteins zipper together in the same membrane, which further pulls the membranes closer.
The lipid bilayers of the membranes mix.
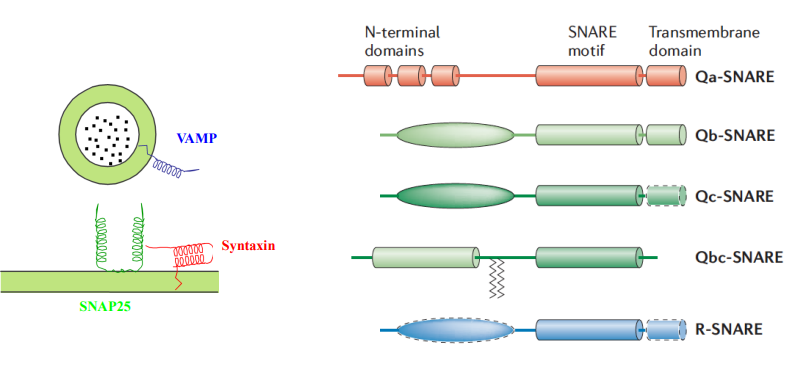
What are R SNAREs, and how do they function in SNARE complexes?
Type of SNARE protein that contain an arginine (R) residue in their SNARE motif.
They play a crucial role in mediating vesicle fusion by interacting with Q SNAREs from target membranes, forming the trans-SNARE complex necessary for membrane docking and fusion.
What are Q SNAREs, and what role do they play in SNARE complexes?
SNARE proteins characterized by the presence of glutamine (Q) residues in their SNARE motifs.
They partner with R SNAREs in a 3:1 ratio to facilitate membrane fusion, ensuring the specificity and efficiency of vesicle transport.
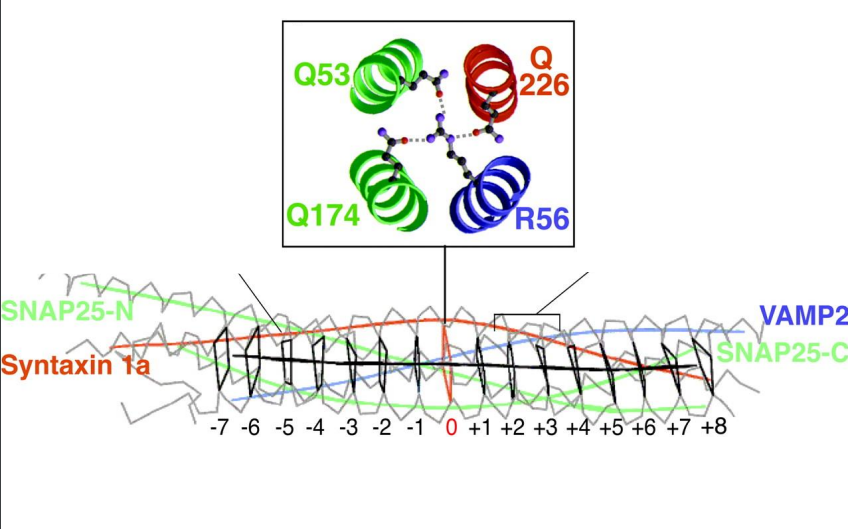
What is the significance of the 3:1 ratio of Q SNAREs to R SNAREs in SNARE complexes?
The 3:1 ratio of Q SNAREs to R SNAREs is conserved in all SNARE complexes, providing a stable and effective arrangement for membrane fusion.
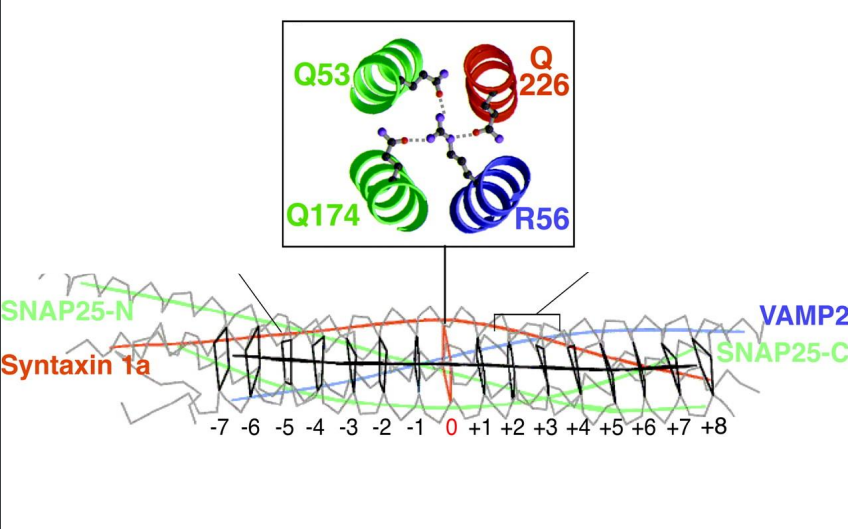
What happens if a Q or R SNARE is mutated?
Mutations in either a Q SNARE or an R SNARE inhibit SNARE activity, disrupting the processes of vesicle fusion and transport, which can affect cellular communication and function.
Do SNAREs provide specificity for membrane fusion?
Yes, SNAREs provide specificity for membrane fusion by forming complexes with a 3:1 ratio of Q SNAREs to R SNAREs. This specific ratio ensures that only compatible SNARE complexes can mediate membrane fusion.
How do SNAREs exhibit promiscuity in their interactions?
While SNAREs predominantly interact with SNAREs from the appropriate membranes, they do show some promiscuity. This means that certain SNAREs can potentially engage with non-specific partners, but they generally maintain specificity for their intended target membranes.
What additional factors contribute to the specificity of membrane fusion beyond SNAREs?
Rabs (small GTPases), coat proteins, and tethering proteins.
These components help ensure that vesicles fuse only with the correct target membranes.
What is the typical size range of SNARE proteins?
Small, typically ranging from 14 to 40 kDa in molecular weight.
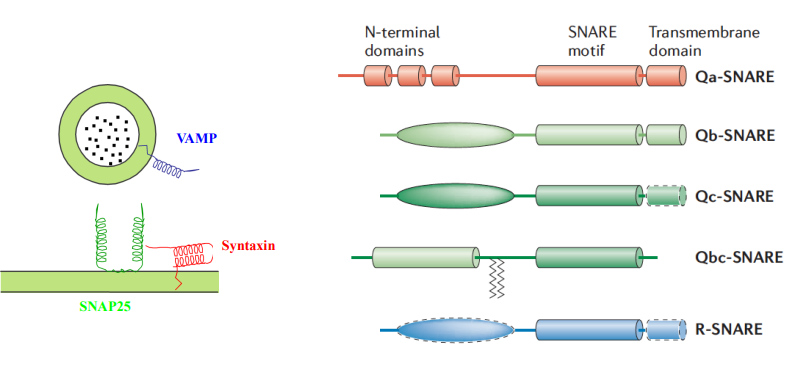
What structural motif do all SNARE proteins possess?
All SNARE proteins contain at least one coiled-coil motif or SNARE motif.
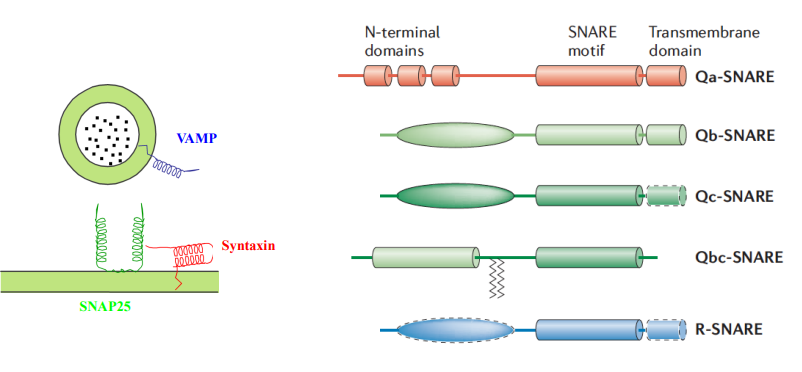
How are SNARE proteins typically anchored in the membrane?
C-terminally anchored, meaning their C-terminus is embedded in the membrane.
Are SNAREs considered the minimal fusion machinery, and how is TIRF microscopy related?
Yes, SNAREs are considered the minimal fusion machinery required for membrane fusion, as they are sufficient to drive the process when properly arranged.
Total Internal Reflection Fluorescence (TIRF) microscopy is a technique used to study SNARE-mediated fusion events in real time by providing high-resolution images of vesicle interactions with target membranes, thereby demonstrating the essential role of SNAREs in membrane fusion dynamics.
What role do recombinant SNAREs play in the fusion of purified liposomes?
Recombinant SNAREs can drive the membrane fusion of purified liposomes even in the absence of calcium. This indicates that SNAREs are sufficient for fusion when properly assembled.
How does calcium affect the fusion rate of liposomes mediated by SNAREs?
The presence of calcium increases the fusion rate of liposomes mediated by SNAREs by about 30-50 fold, enhancing the efficiency of membrane fusion.
What happens to membrane fusion when SNAREs are removed or cleaved with toxins?
Removal of SNAREs or cleavage of SNAREs with toxins results in no fusion of liposomes, even in the presence of calcium.
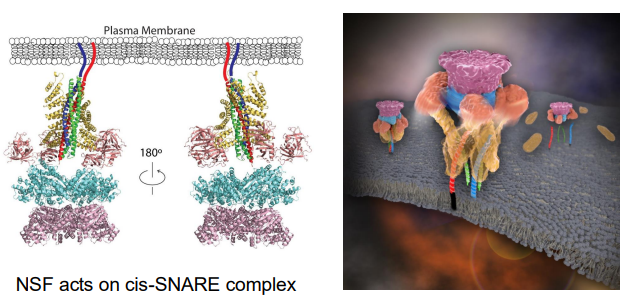
What is the role of NSF in relation to SNAREs, and is it required for the fusion step?
The structure of the SNARE/NSF complex has been solved, revealing that NSF acts specifically on the cis-SNARE complex. NSF facilitates the disassembly of this complex after membrane fusion, allowing the individual SNARE proteins to be recycled for future fusion events.
However, NSF is not required for the fusion step itself; its role is primarily in disassembling SNARE complexes and preparing them for reuse in subsequent fusion events.
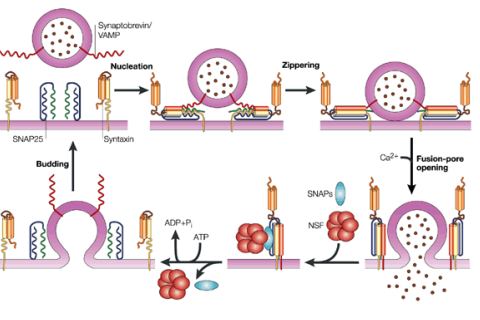
How is SNARE function regulated? (4)
Calcium Levels: Calcium can enhance SNARE-mediated fusion rates.
Accessory Proteins: Can modulate SNARE function by responding to calcium changes.
Post-Translational Modifications: Phosphorylation or ubiquitination can alter SNARE activity or stability.
Disassembly by NSF: NSF facilitates the recycling of SNAREs after fusion, maintaining their availability for subsequent rounds of vesicle trafficking.
How are SNAREs trafficked to the correct membranes? (3)
Sorting Signals
Vesicular Transport: packaged into transport vesicles that move along cytoskeletal filaments to their target membranes.
Rab GTPases: help identify and guide vesicles to their correct destinations, ensuring that SNAREs reach the right membranes for fusion.