Rate of Reaction
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
how does surface area affect rate of reaction?
increase in surface area increases rate of reaction
more of reactant is exposed
more frequent collisions
more successful collisions per unit time
how does concentration affect rate of reaction?
increasing concentration increases rate of reaction
more particles in the same volume
more frequent collisions
more successful collisions per unit time
how does pressure affect rate of reaction?
increasing pressure increases rate of reaction
same number of particles in a smaller volume
more frequent collisions
more successful collisions per unit time
how does temperature affect rate of reaction?
increasing temperature increases rate of reaction
particles have more kinetic energy
more particles collide with the required activation energy
more successful collisions per unit time
how does catalyst affect rate of reaction?
provides an alternative pathway
with a slower activation energy
more successful collisions per unit time
rate of reaction increases
explain how you would be able to re-use the same catalyst later on
as it increases rate without getting used up
name two catalysts
manganese (IV) oxide
lead (IV) oxide
2H2O2 →
O2 + 2H2O
what is the relationship between rate and concentration?
rate is directly proportional to concentration
is rate proportional to temperature?
no
how would you prove the catalyst didn’t get used up in the experiment?
add x grams of catalyst to reaction
filter off catalyst at end
allow to dry
re-weigh catalyst
should still have x grams
why does rate decrease during a reaction?
the reactant gets used up
SA/concentration of reactant decreases
less frequent collisions
less successful collisions per unit time
eventually reaction stops when one reactant is used up
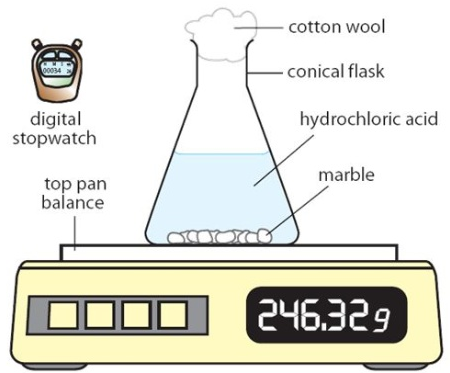
what is the purpose of the cotton wool?
allows gas to escape but prevents liquid escaping
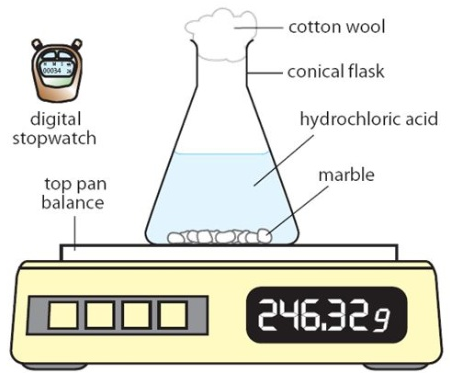
what is going to happen to the reading on the balance during the reaction? Explain.
decrease, as CO2 is produced and escapes through cotton wool
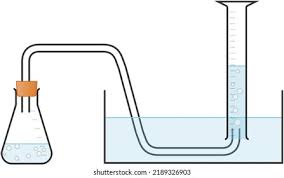
why is this method not that accurate?
hard to read measuring cylinder
CO2 will dissolve in water so lower than expected volume collected
what is a more accurate piece of equipment than using the water measuring cylinder method to collect gas?
gas syringe
what is the test for oxygen?
put glowing splint in the gas
if relights, oxygen in produced
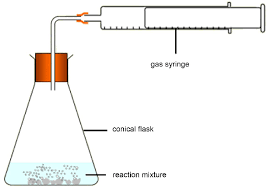
why is this method more accurate than the water cylinder method?
easier to read scale and no gas can dissolve in water
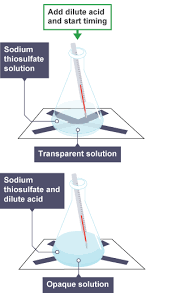
what is the biggest issue with accuracy in this experiment?
human judgement required
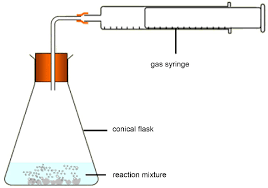
suggest a reason why the volume of gas collected is less than the expected value
gas escaped before bung was put on so reaction started before bung was put on