Week 4: Biomedical Sciences (Haskell-Luevano)
1/107
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
108 Terms
How is glycolysis defined?*
The sequence of reactions that metabolizes one molecule of glucose to two molecules of pyruvate, with a net production of two ATP
Is glycolysis aerobic or anaerobic?
It is an anaerobic process (does not require oxygen), though subsequent reactions may depend on O₂ availability
What is the net ATP yield of glycolysis?
2 ATP per glucose molecule
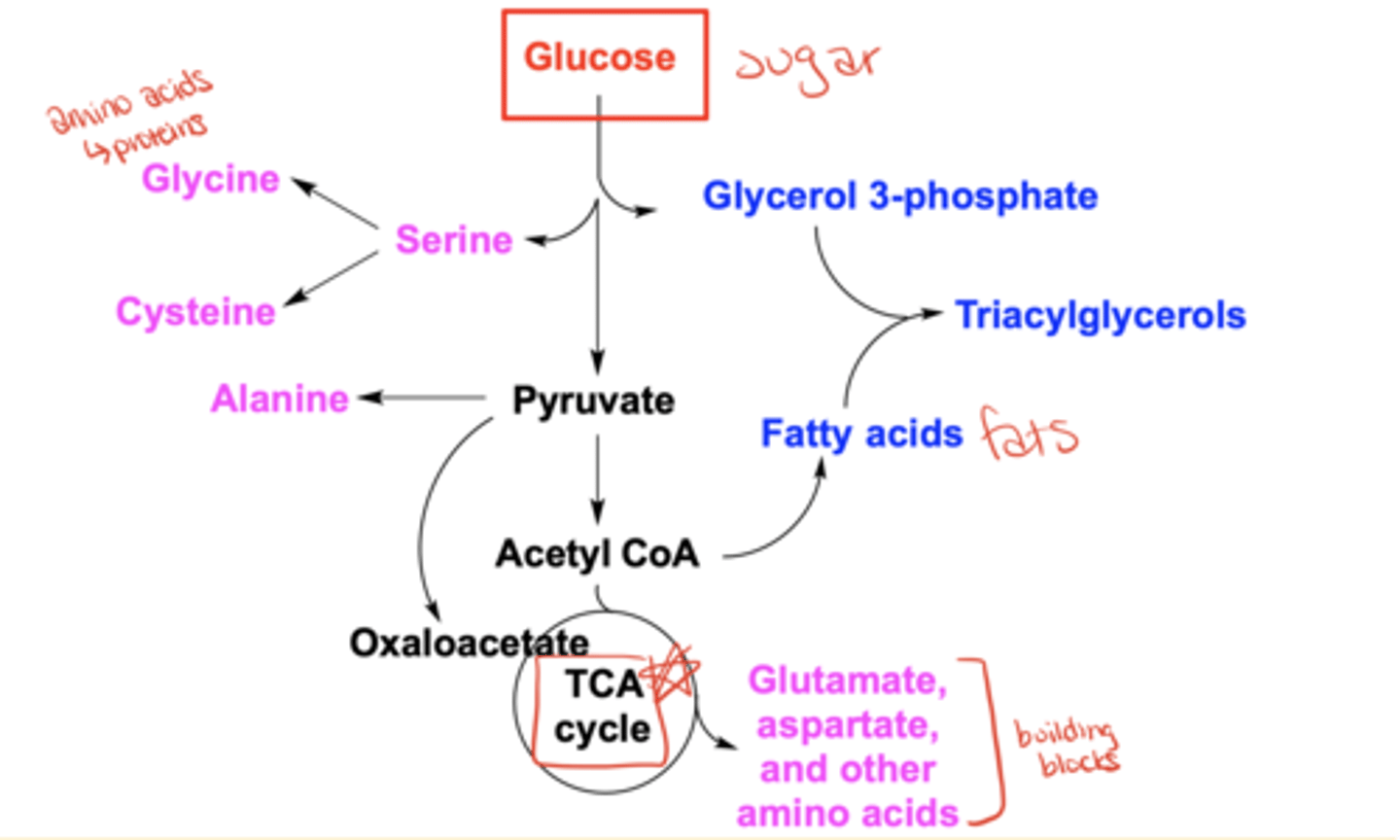
What are some possible fates of glucose besides glycolysis?
It can be converted into amino acids (e.g., glycine, serine, alanine), glycerol 3-phosphate, fatty acids, triacylglycerols, acetyl-CoA, oxaloacetate, or enter the TCA cycle
What percent of caloric intake in the US diet comes from carbohydrates?
~40-45%
How much glucose does the brain require per day?
~120 grams/day
Why is glucose called the "universal fuel"?
Because every human cell type can generate ATP from glycolysis
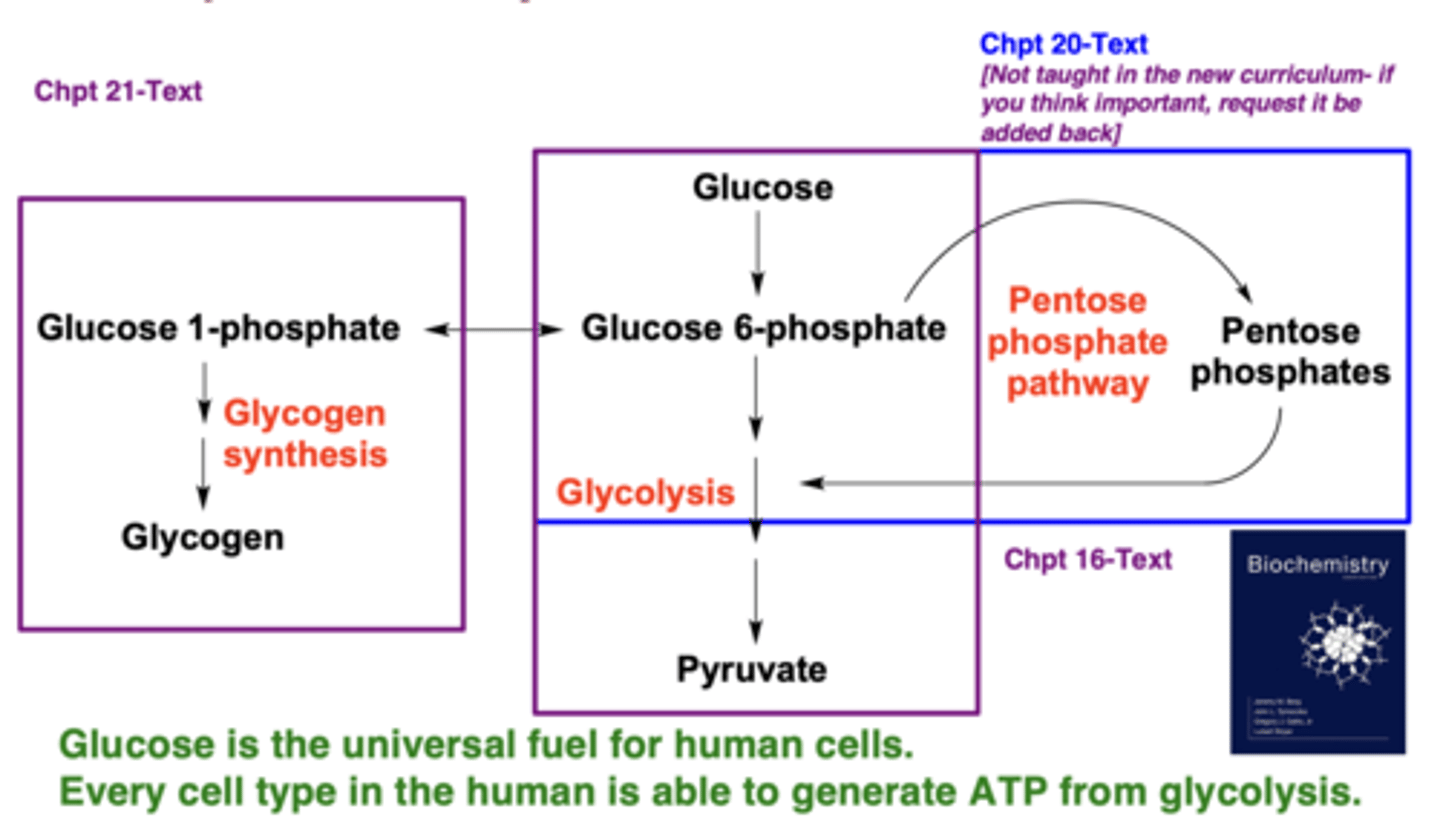
Which pathways metabolize glucose?
Glycolysis, glycogen synthesis/breakdown, and the pentose phosphate pathway
What are three characteristics of glycolysis?
It is nearly universal, anaerobic, and highly controlled
Which tissues depend solely on glucose as a fuel?
The brain (under non-starvation) and red blood cells
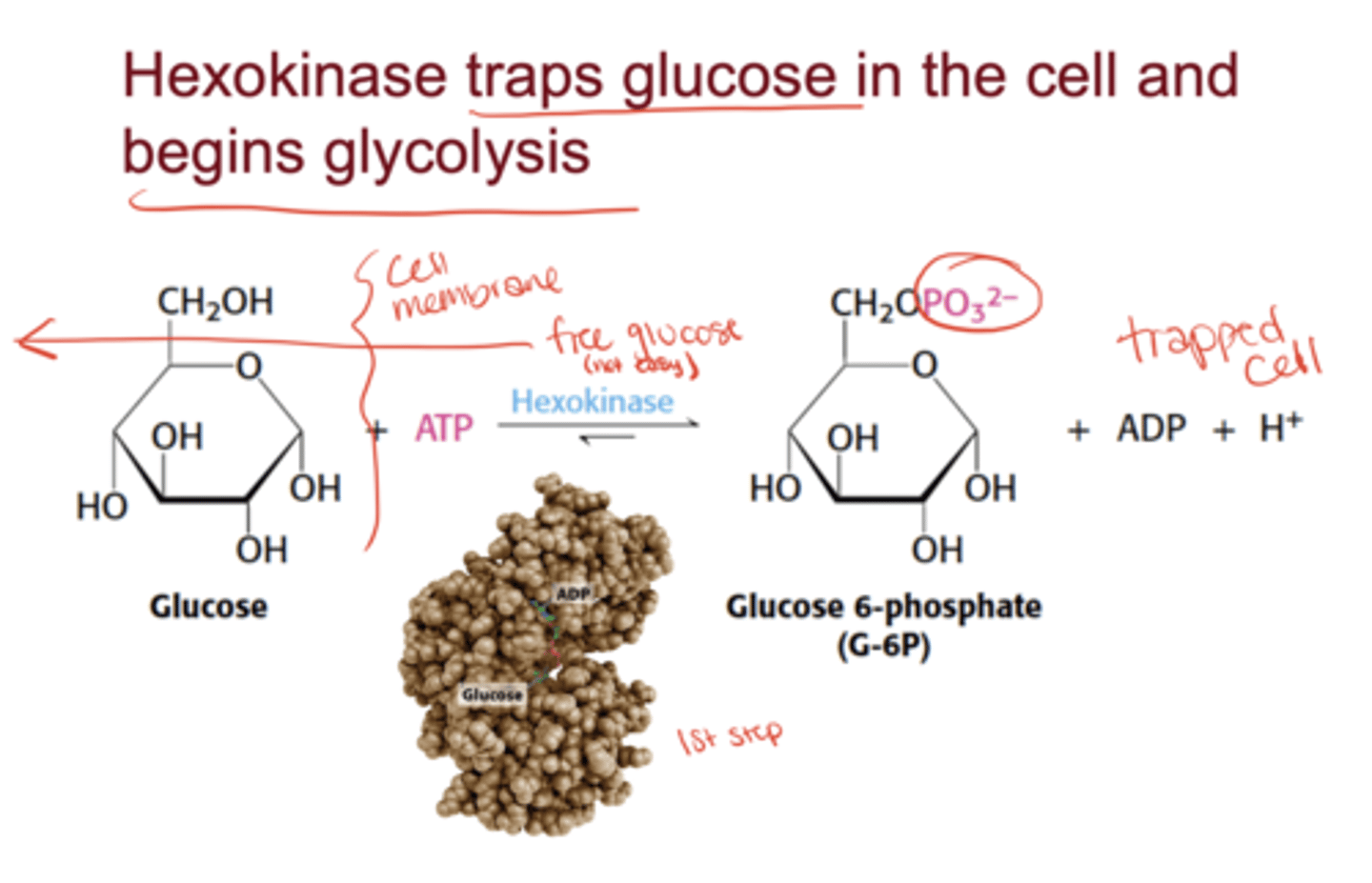
What is the role of hexokinase?
It phosphorylates glucose to glucose-6-phosphate, trapping it inside the cell and beginning glycolysis
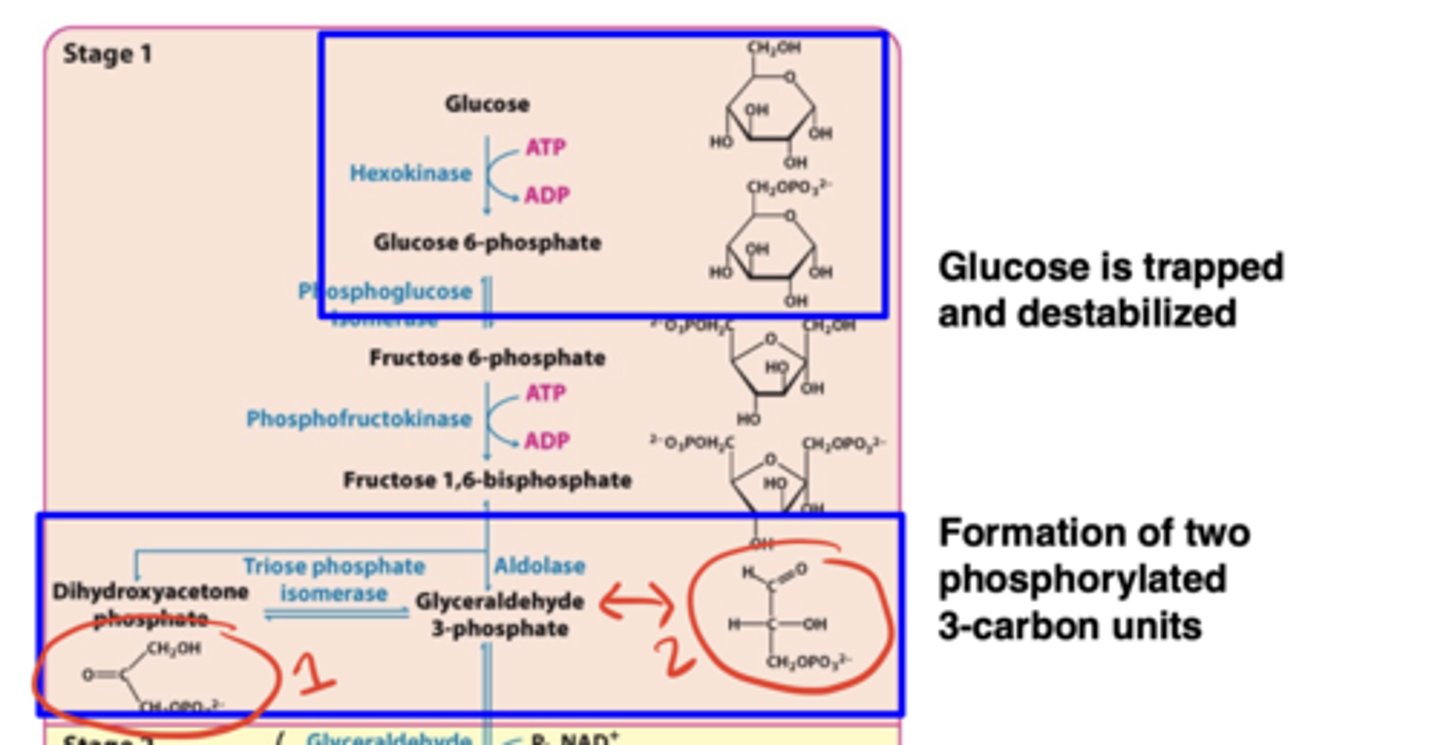
What happens in Stage 1 of glycolysis?
Glucose is trapped and destabilized; ATP is consumed; fructose-1,6-bisphosphate is cleaved into two phosphorylated 3-carbon units
What happens in Stage 2 of glycolysis?*
Oxidation of the 3-carbon fragments to pyruvate and harvesting of ATP (net +2 ATP)
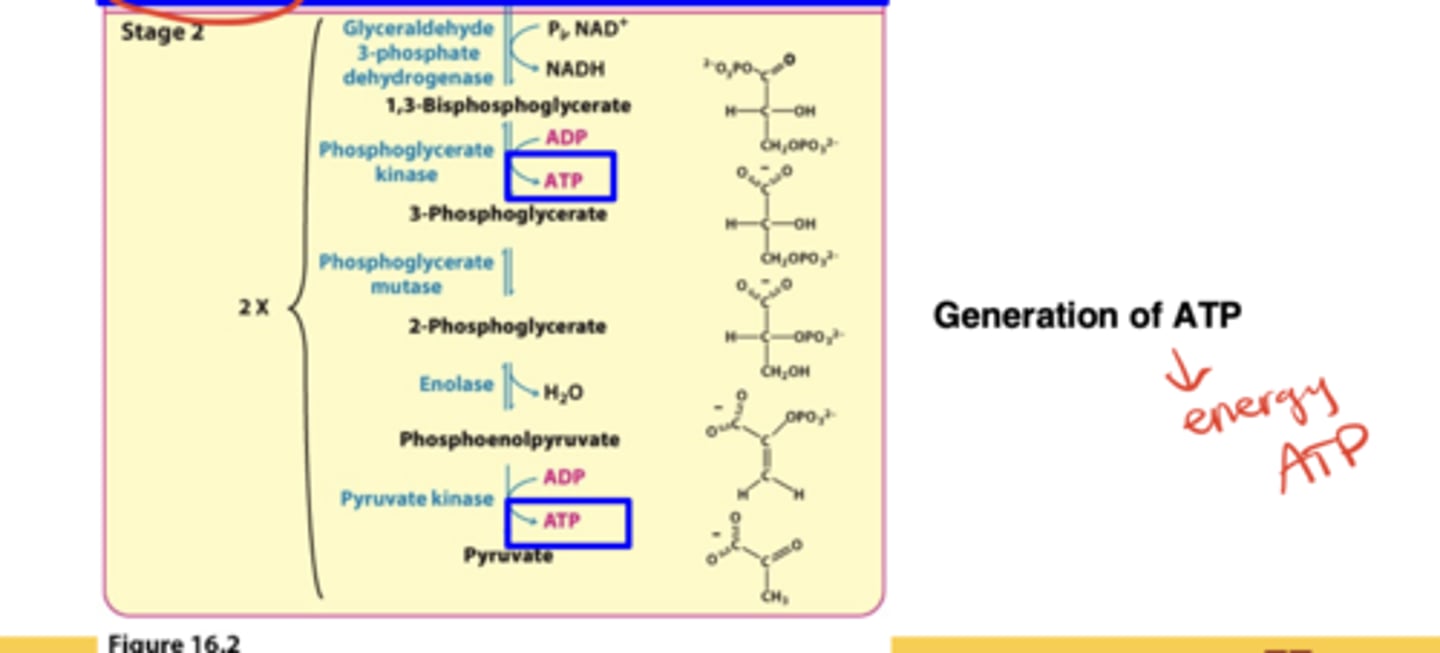
What are the two main cellular needs glycolysis meets?
Production of ATP and production of building blocks
Which three enzymes are irreversible and serve as glycolysis control sites?*
Hexokinase, phosphofructokinase (PFK), and pyruvate kinase
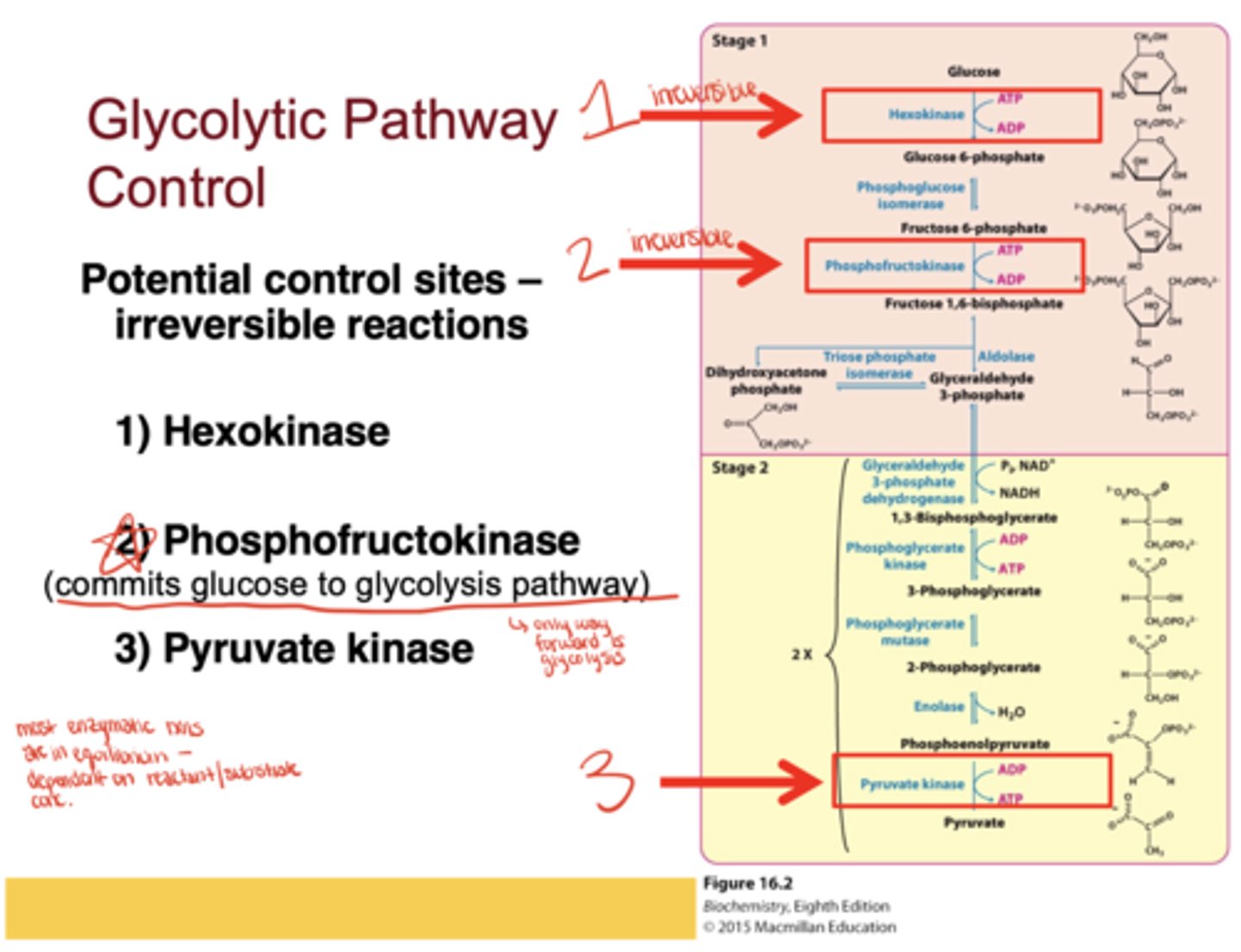
Which enzyme is considered the most important control point in glycolysis?*
Phosphofructokinase, because it commits glucose to the glycolytic pathway
How do GLUT transporters move glucose across membranes?
Glucose binding changes the transporter's conformation, releasing glucose inside the cell, after which it resets for another cycle
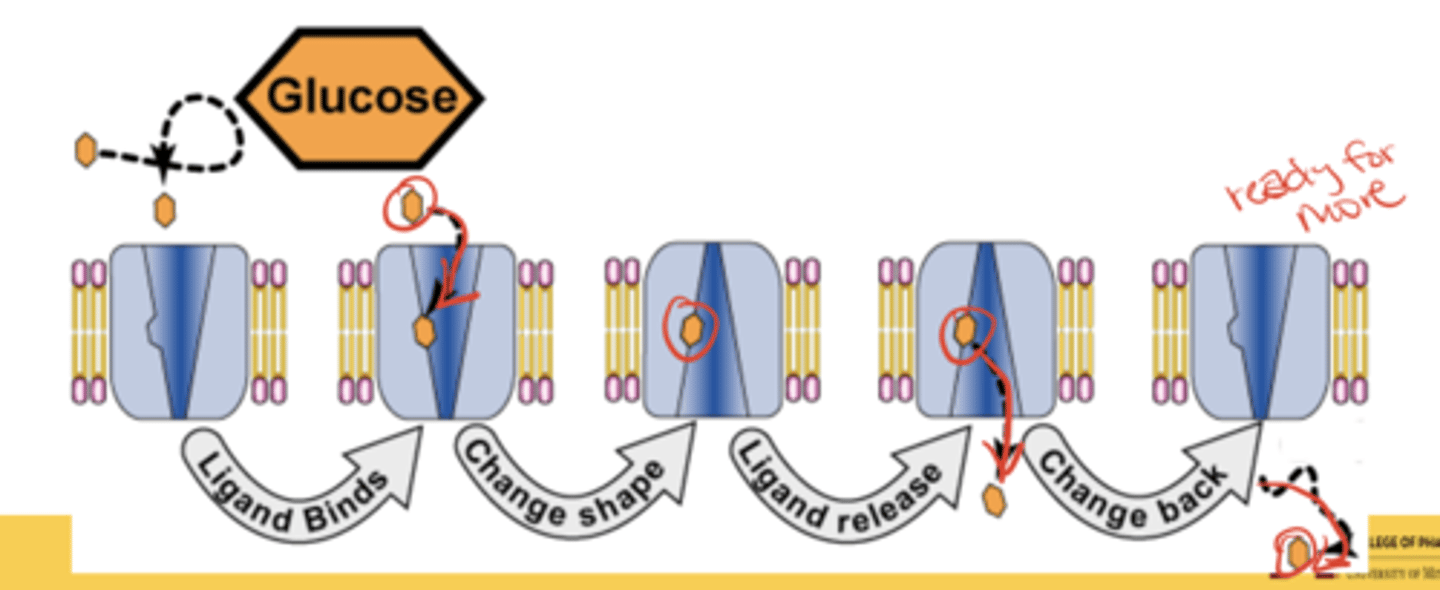
Are GLUT transporters active or facilitated transporters?
Facilitated transporters
- glucose moves down its concentration gradient without expending metabolic energy
How many GLUT transporters are encoded in the human genome?
12, each with distinct tissue distribution and kinetic properties
What is Km?
The substrate concentration at which half of enzyme active sites are filled; reflects enzyme-substrate affinity
Which GLUTs are responsible for basal glucose uptake?*
GLUT1 and GLUT3
Where is GLUT1 found?
Erythrocytes and blood-brain barrier cells
Where is GLUT3 found?*
Neurons of the CNS
Which GLUTs handle basal glucose uptake?*
GLUT1 and GLUT3
What happens when blood glucose falls to 1-3 mM?
Slowed transport across the blood-brain barrier causes hypoglycemia (light-headedness, dizziness, coma if untreated)
Which GLUT functions only when blood glucose is elevated?*
GLUT2
Where is GLUT2 found and what is its role?
In liver and pancreatic β-cells; it removes excess glucose from blood and helps regulate insulin secretion
- High Km means it works mainly after carbohydrate-rich meals
Where is GLUT4 found and how is it regulated?*
In muscle and fat cells; it is regulated by insulin
Where is GLUT5 found and what does it transport?
In the small intestine; transports fructose (fruit sugar)
How does glucose trigger insulin release in pancreatic β-cells?
1. Glucose enters via GLUT2.
2. Glucokinase converts it to G-6-P.
3. Glycolysis, TCA, and oxidative phosphorylation increase ATP.
4. ATP closes ATP-sensitive K⁺ channels → depolarization.
5. Ca²⁺ influx triggers exocytosis of insulin vesicles
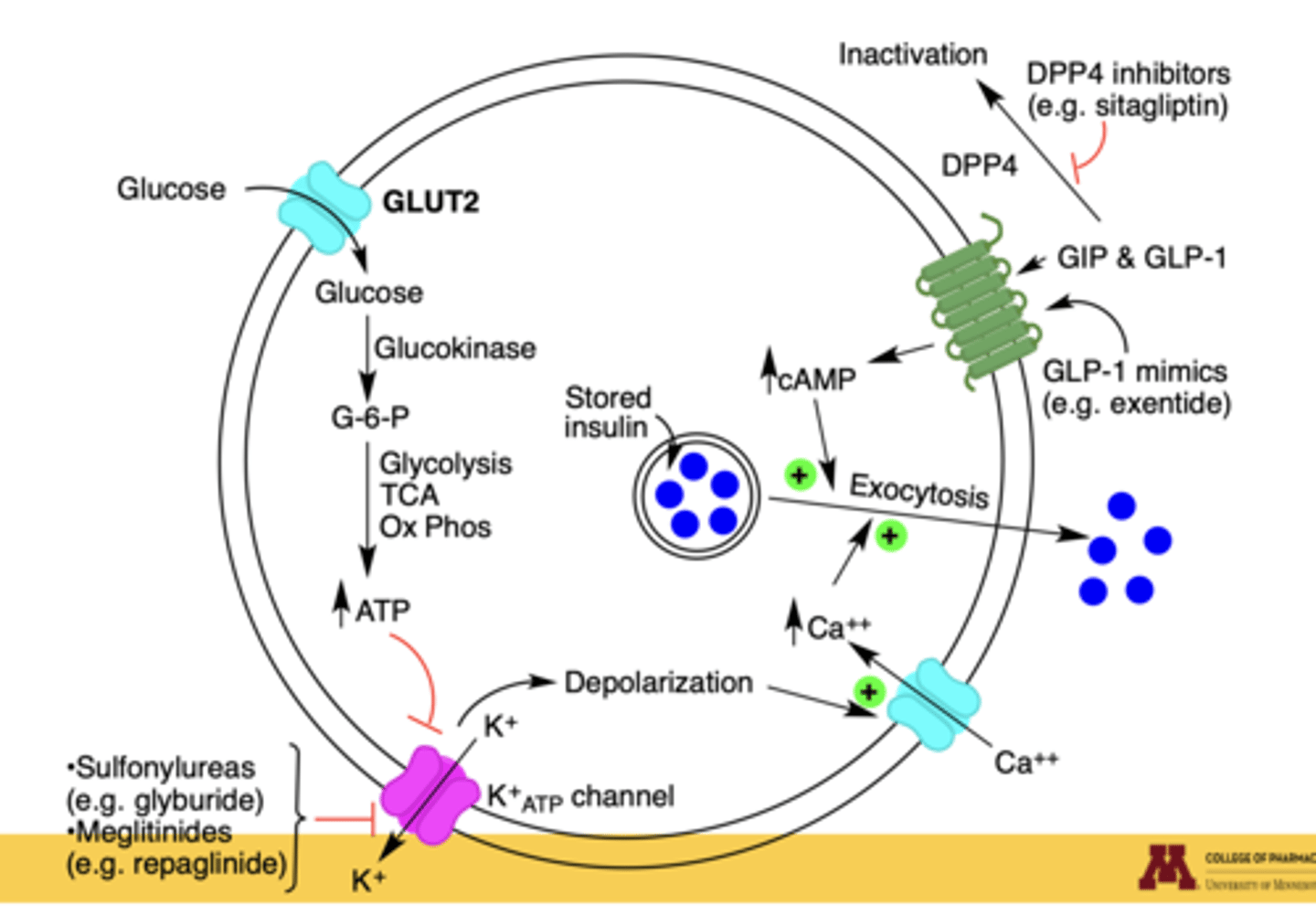
Which diabetes drugs enhance or mimic this process?
Sulfonylureas (glyburide), meglitinides (repaglinide) → close K⁺ channels
GLP-1 mimics (exenatide) and GIP → increase cAMP, enhancing insulin secretion
DPP-4 inhibitors (sitagliptin) → prolong incretin action
How does insulin regulate GLUT4?
Insulin signaling mobilizes GLUT4 vesicles to the plasma membrane, allowing glucose uptake into muscle and fat
What happens in type 1 diabetes regarding GLUT4?
Lack of insulin release prevents GLUT4 mobilization, leading to low glucose uptake and prolonged hyperglycemia
What happens in type 2 diabetes or insulin resistance regarding GLUT4?
Decreased insulin receptors and impaired GLUT4 trafficking reduce glucose transport; adipocytes also show decreased GLUT4 gene expression
How is diabetes defined?
A heterogeneous disease with syndromes characterized by:
- hyperglycemia (↑ blood sugar)
- altered metabolism of lipids, carbohydrates, and proteins
- increased risk of vascular disease complications
What factors influence diabetes prevalence?
Sex at birth, age (higher prevalence with older age), and ethnicity (risk differences due to both genetics and life experiences)
What are the three main types of diabetes?
Type 1 Diabetes (T1D), Type 2 Diabetes (T2D), and Gestational Diabetes (GDM)
What does it mean that diabetes is a heterogeneous disease?
It can result from mutations in one or multiple genes, life events, environmental exposures, or endocrine disorders
Which key enzyme mutation can predispose to diabetes?*
Glucokinase (hexokinase IV), which phosphorylates glucose to glucose-6-phosphate, trapping it in the cell
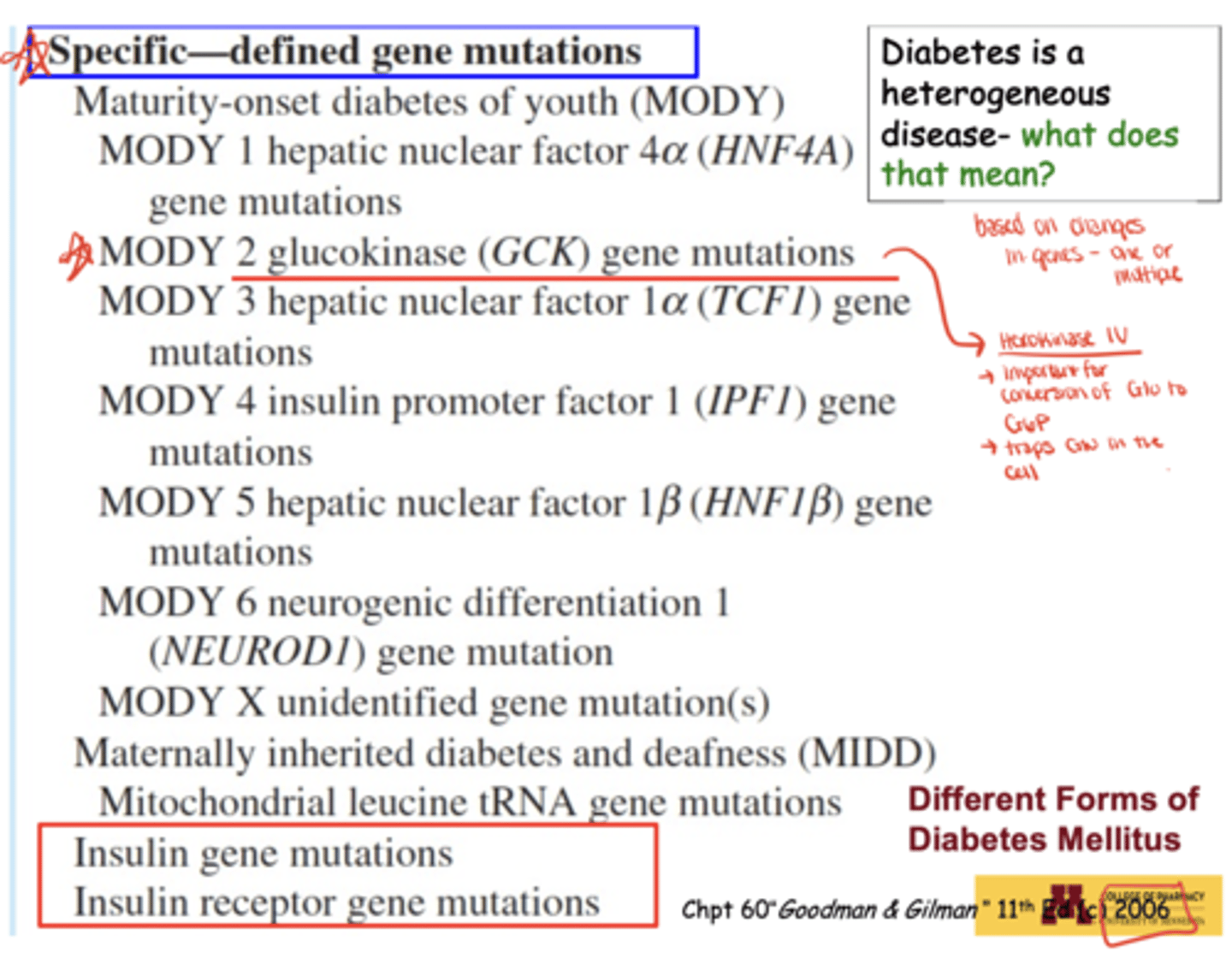
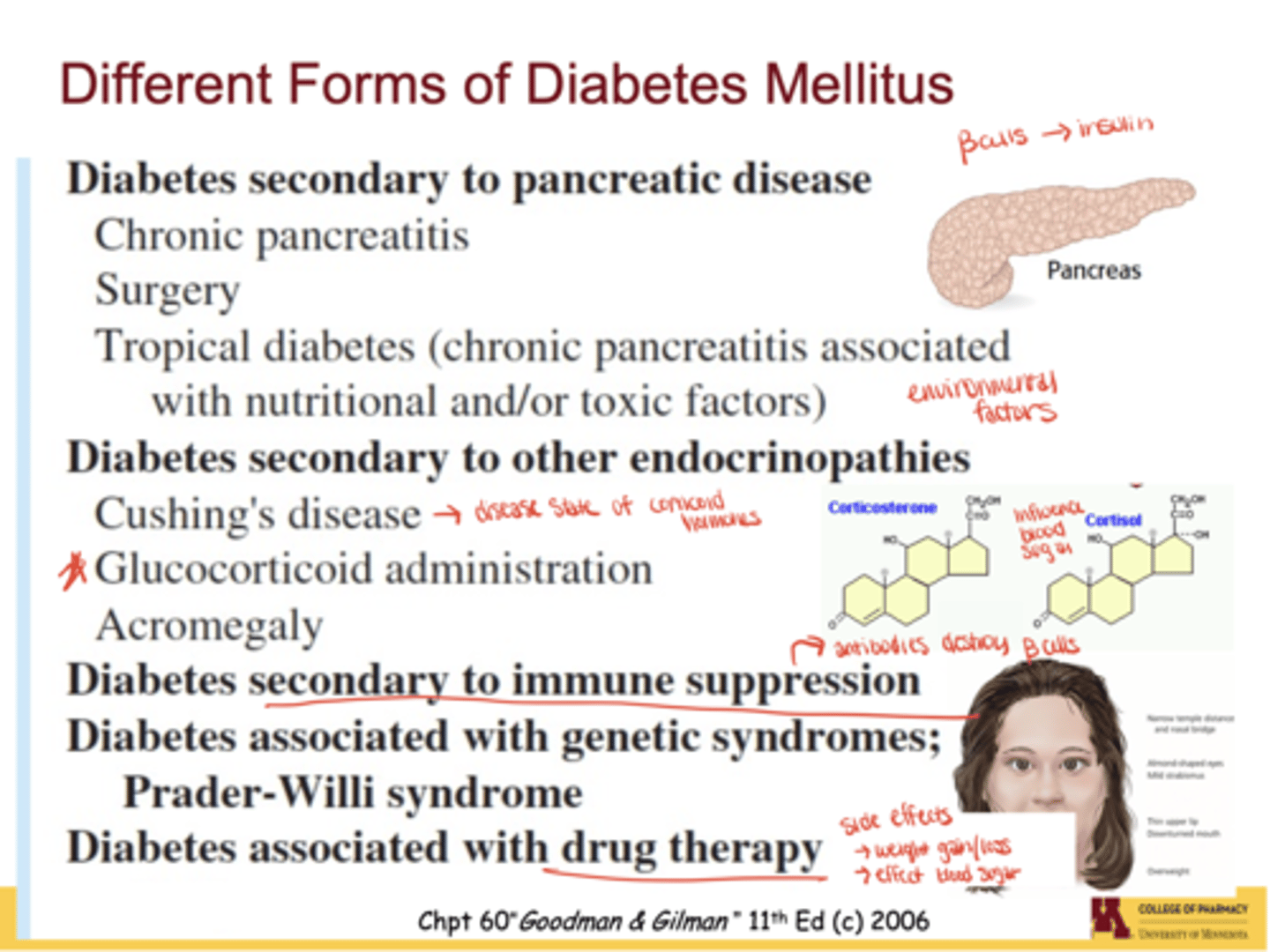
What environmental or medical factors can trigger diabetes?
Chronic pancreatitis, pancreatic surgery, nutritional deficiencies ("tropical diabetes"), toxins/pesticides, endocrinopathies (Cushing's, acromegaly), steroid drugs (cortisol, corticosterone), and immune suppression
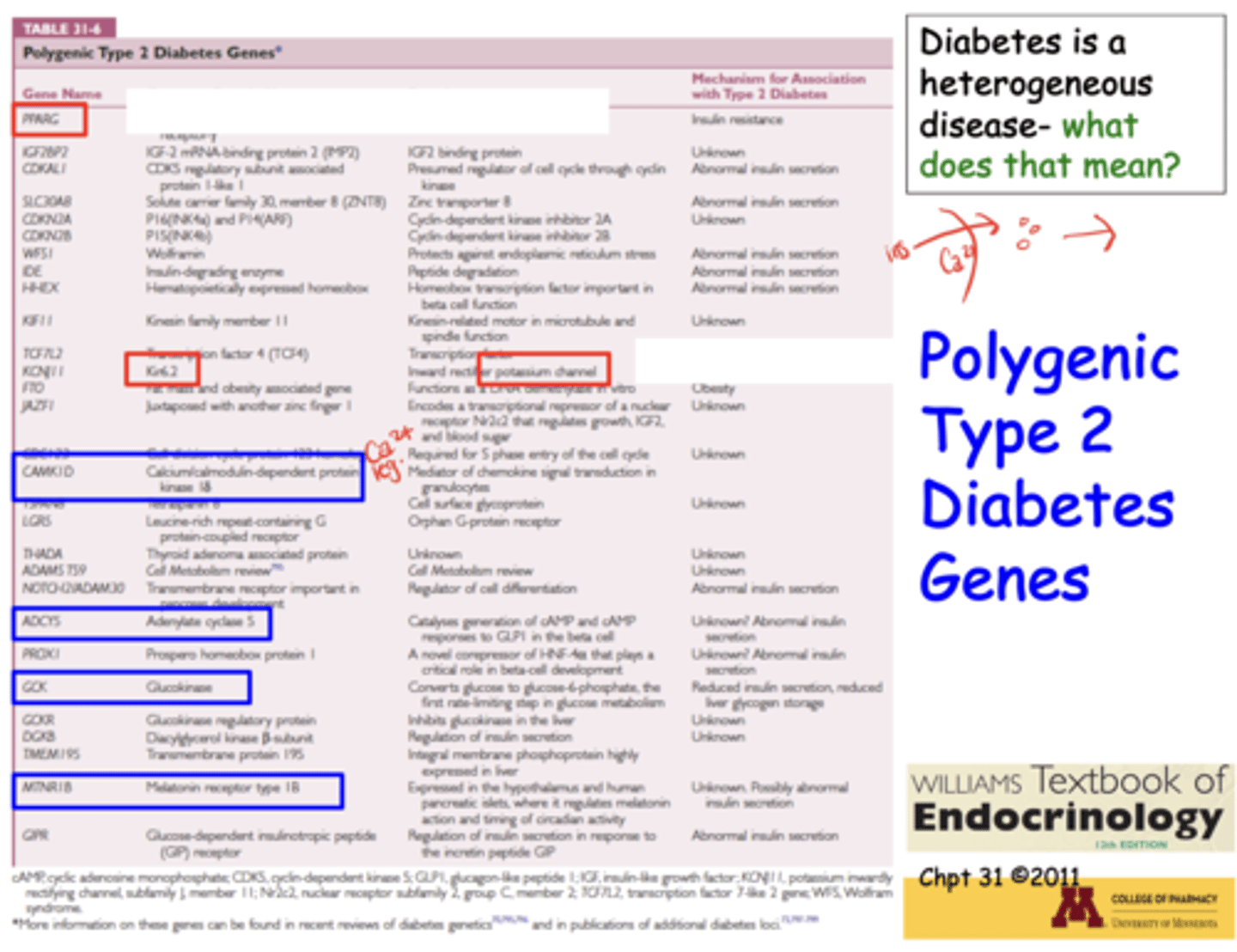
Which genes are linked to Type 2 diabetes?*
What are the diagnostic criteria for diabetes?
- Random plasma glucose ≥ 200 mg/dL with symptoms
- Fasting plasma glucose ≥ 126 mg/dL
- 2-hr OGTT glucose ≥ 200 mg/dL
- A1C ≥ 6.5
What are common symptoms of diabetes?
Polyuria (frequent urination), polydipsia (excessive thirst), unexplained weight loss
Which key pathways regulate blood glucose?
Glycolysis, glucose transporters (GLUTs), gluconeogenesis, glycogen breakdown/synthesis, metabolic syndrome, and hormones (insulin, glucagon, epinephrine, cAMP)
What are the primary tissues and organs affected by diabetes?*
- Pancreas (β-cells)
- Liver
- Skeletal Muscle
- Adipose Tissue
- Kidneys
- Nervous System
- Eyes
- Cardiovascular System
What are normal serum glucose levels?
4-8 mM (72-144 mg/dL)
How many Americans have diabetes?
~29.1 million (~9.3% of population
~1/3 undiagnosed
What proportion of diabetics are Type 2 vs Type 1?
90% are Type 2; 5-10% are Type 1
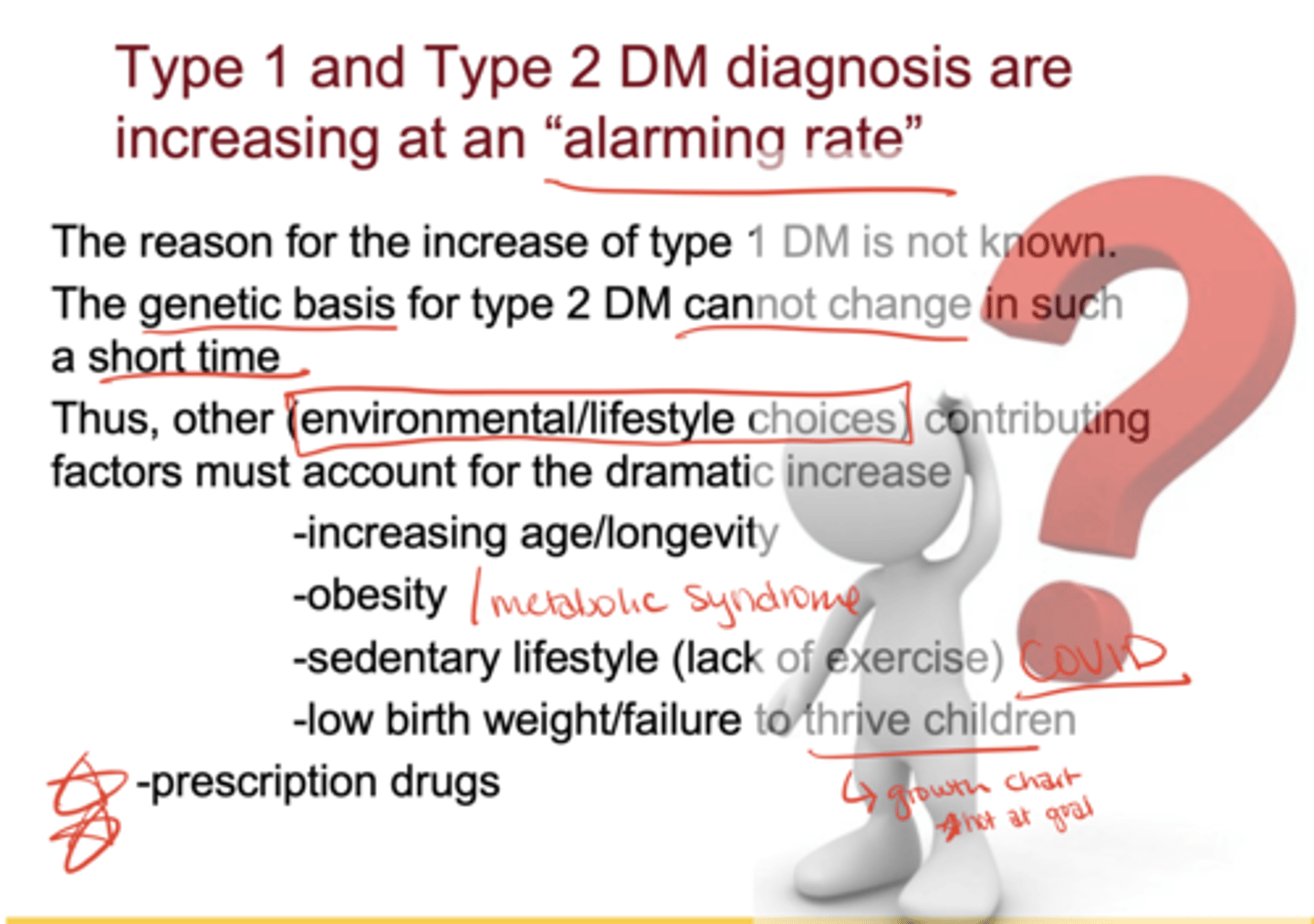
Why are Type 1 and Type 2 diabetes rates rising?*
Type 1: cause unknown
Type 2: not genetics (too fast to change), but environment/lifestyle factors: ↑ longevity, obesity, sedentary lifestyle, low birth weight/failure to thrive, prescription drugs
What causes Type 1 diabetes?*
Autoimmune destruction of pancreatic β-cells → absolute insulin deficiency
What are key characteristics of Type 1 diabetes?*
Onset <30 yrs (peak 12-14 yrs)
Prevalence: 0.5% of general population, 5-10% of diabetics
Non-obese phenotype
Low genetic concordance (40-50% in identical twins)
What are the acute and chronic complications of T1D?*
Acute: hyperglycemia, ketoacidosis, wasting
Chronic: neuropathy, retinopathy, nephropathy, PVD, CHD
What lab marker correlates with insulin secretion?
C-peptide (byproduct of insulin biosynthesis)
Is insulin required for management of T1D?*
Yes — absolutely required
What are key characteristics of Type 2 diabetes?*
- Onset usually >40 yrs (but increasingly in children)
- Prevalence: 6-10% general population, ~90% of diabetics
- 60-90% obese
- High genetic concordance (95-100% in identical twins)
Is ketoacidosis common in T2D?
rare
What are the causes of T2D?*
Decreased insulin secretion, insulin resistance, strong genetic component, polygenic risk
Is insulin required for T2D management?*
No, lifestyle modification and hypoglycemic agents are first-line; insulin may be added
What are the metabolic characteristics of diabetes?*
- impaired glucose transport (muscle, fat)
- impaired glucose utilization (muscle, fat, liver)
- ↓ glycogenesis
- ↓ glycolysis
- ↑ glucose production in liver
What is A1C?*
Glycosylated hemoglobin, a measure of % HbA irreversibly bound to glucose
Why is A1C a clinical indicator?*
It reflects average blood glucose over ~3 months (RBC lifespan) and predicts long-term complications, especially microvascular
What are the microvascular complications of diabetes?*
Retinopathy, nephropathy, neuropathy
Why do microvascular complications develop?
Glucose toxicity and reactive oxygen species damage tissues with high glucose susceptibility
What are the macrovascular complications of diabetes?*
Stroke, coronary artery disease, peripheral vascular disease
What causes macrovascular complications?
Multifactorial
- less dependent on hyperglycemia, more on insulin resistance, hyperinsulinemia, hypertension, dyslipidemia, platelet hypersensitivity
What is the source of insulin (organ/cell type)?
Insulin is produced in the pancreas, specifically by the β-cells of the islets of Langerhans.
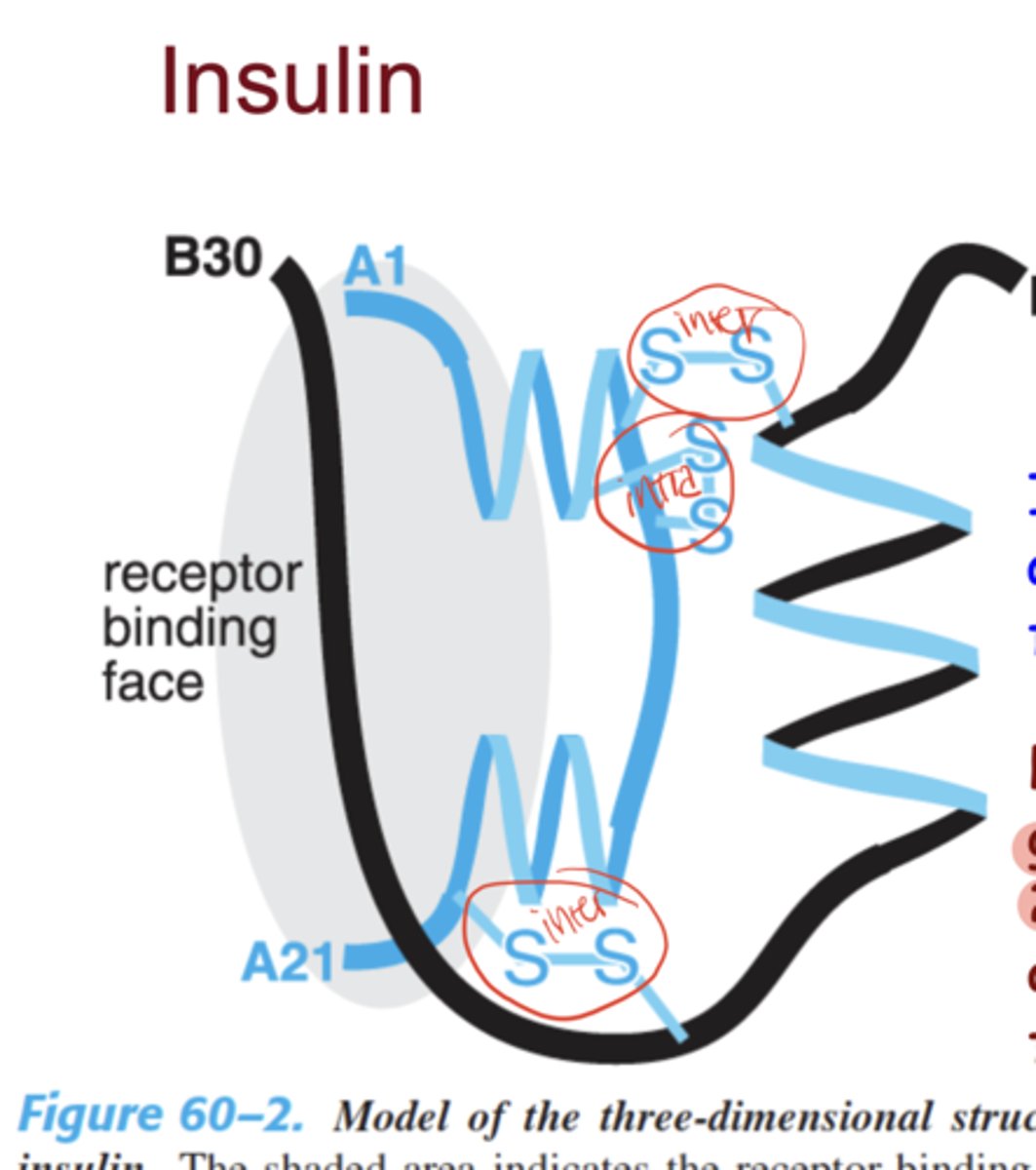
How many peptide chains make up insulin?
Composed of two peptide chains (A-chain = 21 amino acids, B-chain = 30 amino acids)
What type of bonds exist between insulin peptide chains and where?
Linked by two inter-chain disulfide bonds (A and B) + one intra-chain bond in the A-chain
How is insulin synthesized?
synthesized as preproinsulin → proinsulin → insulin + C-peptide
Insulin Receptor is
heterotetrameric transmembrane glycoprotein
What is the insulin receptor composed of?
Composed of 2 extracellular α-subunits (ligand-binding) and 2 transmembrane β-subunits (tyrosine kinase activity
What is the result of insulin receptor activation?
leads to autophosphorylation and intracellular signaling cascades (PI3K/Akt, MAPK)
Insulin can exist as how many typical subunits (multimers)?
monomers, dimers, and hexamers
What is unique about hexamer forms of insulin?
(stabilized by zinc) and are the storage form in pancreatic granules and in pharmaceutical insulin preparations
What are the circulating half-lives of insulin & proinsulin?
Insulin: ~5–6 minutes.
Proinsulin: ~30 minutes (longer because it is less rapidly cleared)
What is C-peptide used for clinically?
- to assess endogenous insulin secretion (since exogenous insulin does not contain C-peptide)
Why is C-peptide useful for diagnosing diabetes?
useful for distinguishing type 1 vs type 2 diabetes and assessing β-cell function
What are the main inhibitors & stimulants of insulin secretion (classes/types)?
Inhibitors of insulin secretion
sympathetic stimulation (α2-adrenergic agonists like norepinephrine), somatostatin, diazoxide
Stimulants of insulin secretion
glucose (via GLUT2), amino acids, incretins (GLP-1, GIP), parasympathetic (ACh), sulfonylureas
Endocrine
bloodstream to distant target
Paracrine
secrete to nearby cells
ex. β- to α-cells in pancreas
Autocrine
self-releases (acts on itself)
Exogenous Delivery
injected insulin formulations
GLUT1 tissue expression
RBCs, brain, basal uptake
GLUT2 tissue expression
Liver, pancreatic β-cells, kidney (bidirectional, high-capacity)
GLUT3 tissue expression
Neurons (high affinity)
GLUT4 tissue expression
Skeletal muscle, adipose tissue (insulin-dependent)
GLUT5 tissue expression
Small intestine (fructose transport)
Why are GLUT2 and GLUT4 important for diabetes?
GLUT2: Critical for glucose sensing in pancreatic β-cells (triggers insulin release).
GLUT4: Major transporter for insulin-stimulated glucose uptake in muscle and adipose tissue; defects lead to insulin resistance.
Insulin MOA
binds to the insulin receptor (RTK) → autophosphorylation → activation of PI3K/Akt and MAPK pathways → ↑ glucose uptake, ↑ glycogen synthesis, ↑ lipid/protein synthesis
How is insulin degraded?
Primarily by insulin-degrading enzyme (IDE) in the liver, kidney, and muscle
Rapid-acting Insulin Duration
Onset 10-20 min, peak 1-3 hrs, duration 3-5 hrs
ex. lispro, aspart, glulisine
Short-acting Insulin Duration
Onset 30-60 min, peak 2-4 hrs, duration 5-8 hrs
ex. regular insulin
Intermediate-acting Insulin Duration
Onset 1-2 hrs, peak 4-12 hrs, duration 12-18 hrs
Long-acting Insulin Duration
Onset 1-2 hrs, relatively flat, duration up to 24 hrs
ex. glargine,detemir
Ultra-long acting Insulin Duration
Onset ~1 hr, no peak, duration >42 hrs
ex. degludec
Metformin targets the _______ and ________ to _____ glucose production
liver/skeletal muscle/ decrease
SGLT2 drugs target the ______ and ______ glucose reabsorption
kidney/decreases (inhibits)
What are the 3 SGLT2 drugs discussed in this lecture?
Canagliflozin (Invokana), Dapagliflozin (Farxiga), and Empagliflozin (Jardiance)