Chemistry Midterm
1/34
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
1000
How many joules in a kilojoule
4
how many sig figs? - 190.0
3
how many sig figs - 4.50 × 10³
0
freezing point of celcius
Graduated Cylinder
?
Beaker
?

Erlenmeyer flask
?

funnel
?

ring stand
?

100
what is the boiling point of water celcius
373K
convert 100 degrees Celsius to kelvin
crucible and cover
?

lab burner
?

endothermic
absorbs energy
exothermic
releases energy
melting, vapor condensing
endothermic example
freezing, boiling
exothermic example
melting, freezing
heat of fusion
evaporation, condensation
heat of vaporization
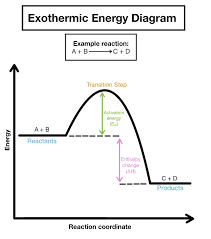
exothermic
?
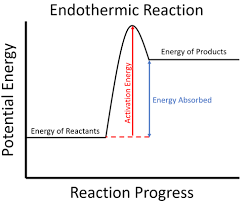
endothermic
?
Q= m * c * DeltaT
equation for specific heat
inverse
pressure and volume have a ___ relationship
direct
volume and temp have a ___ relationship
direct
pressure and temp have a ____ relationship
discovered that all atoms contain tiny negatively charged subatomic particles or electrons.
JJ Thomson
discovering the atomic nucleus, proving that the atom is mostly empty space with a tiny, dense, positively charged center where most of the mass is concentrated,
Ernest Rutherford
most known for discovering the structure of the atom
Niels Bohr