Biological Molecules
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
96 Terms
What is oxytocin?
The first polypeptide hormone to be chemically synthesised. It is used to induce labour.
How does Enfuvirtide inhibit HIV?
It acts by blocking the fusion of viral and host cell membranes.
What does Epoxomicin do and how?
Inhibits the proteasome in the cell as it's epoxide & ketone group react covalently with the active site of the proteasome.
Why are carbohydrates required for cells?
They are used as energy sources, but also cover the cell surfaces and form a sugar coating called the glycocalyx in which they act as:
Receptors for extracellular signals, pathogens and other cells.
A way to mediate communication between cells and their environment.
In what ways can we find out the structures and functions of glycans?
Metabolic glycan labelling.
Cell surface engineering with synthetic carbohydrates.
What is metabolic glycan labelling?
Used to install probes for glycan detection and analysis,
reengineer cell surfaces, or for targeted delivery of cargo.
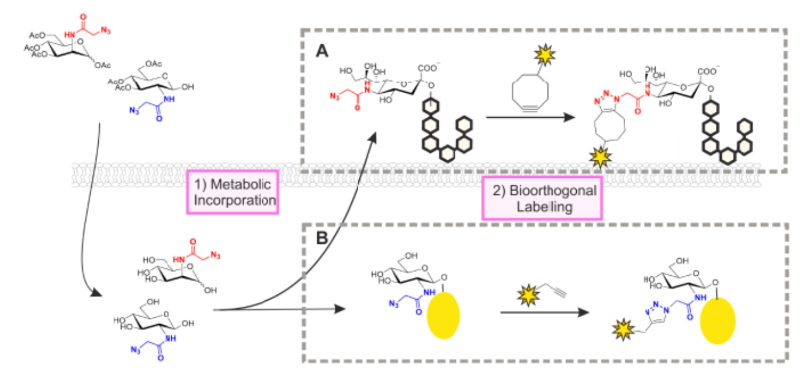
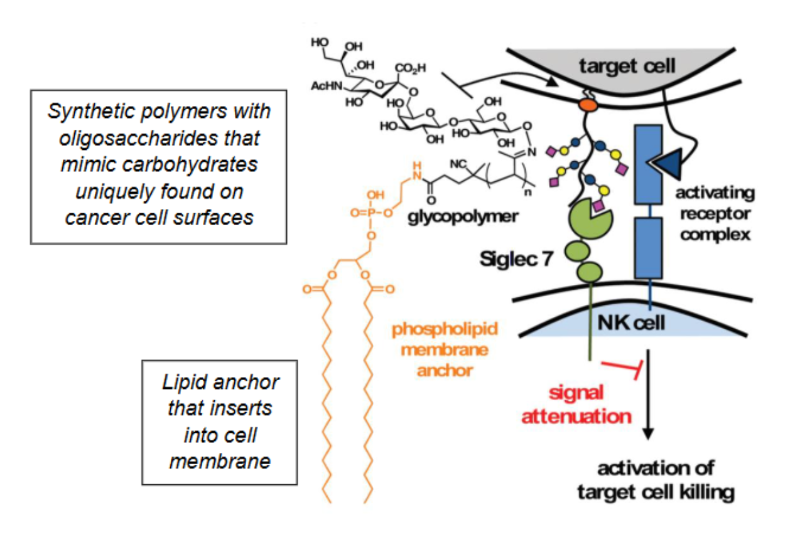
What is cell surface engineering?
Cultured cells are engineered with synthetic carbohydrates to mimic the surface of cancer cells, allowing us to study how the carbohydrates contribute to the binding and activation of immune cells.
What is Acarbose?
A carbohydrate anti-diabetic drug that inhibits enzymes needed for the digestion of complex carbohydrates.
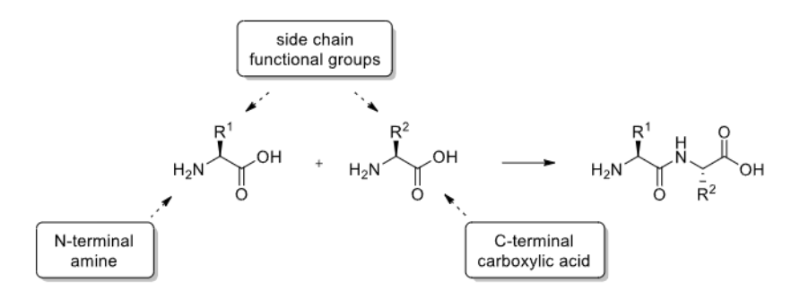
How are peptides structured?
Consist of amino acids linked by peptide bonds via a condensation reaction.
How are peptide sequences notated?
3-letter codes are often used to represent the amino acids.
Peptide sequences are written from the N to C terminus.
Amino acids can be L or D, if it is not indicated it is assumed to be L.
What is a monosaccharide?
A single sugar unit.
What is a disaccharide?
Two sugar units linked together.
What is an oligosaccharide?
More than two sugar units linked together.
What is a glycan?
A general term to describe molecules with glycosidic bonds.
What is a glycoconjugate?
A general term for a carbohydrate covalently linked to another biomolecule.
What is mutarotation?
When free monosaccharides can equilibrate between the acylic and cyclic forms, leading to a mixture of two stereoisomers.
The anomeric hydroxyl group is placed equatorially or axially.
How does mutarotation occur?
The OH in the 1st position forms a double bond to the C, breaking the bond between C1 and the O, forming the acylic form.
The OH in the 5th position then attacks the ketone in the 1st position to reform the cyclic form.
What is the alpha anomer?
C1 & C5 substituents are on opposite sides of the ring.
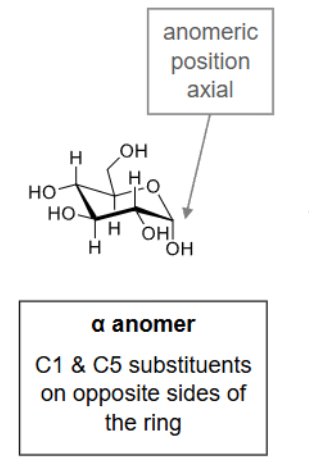
What is the beta anomer?
C1 & C5 substituents are on the same side of the ring.
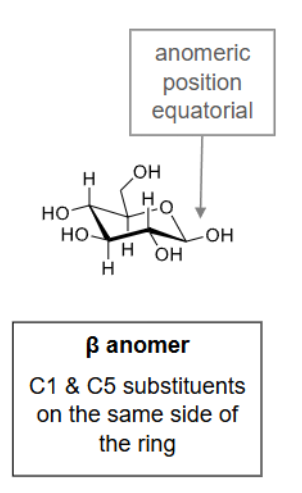
What is the most stable conformation of hexose sugars & why?
The chair conformation:
The equatorial placement is preferred over the axial.
When is the anomeric stereochemistry fixed?
When a glycosidic bond is formed as the R group prevents ring opening.
Mutarotation requires a free hydroxyl group at the anomeric position.
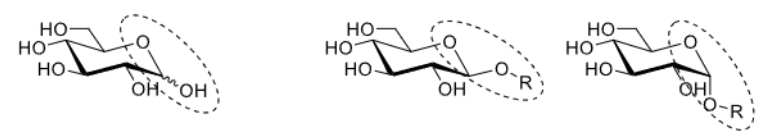
How are glycosidic bonds formed?
Via a condensation reaction.
This is a Gal-alpha 1,6-GlcNAc bond.
How do you increase reactivity of amino acids?
Activate the C-terminal carboxylic acid OH by turning it into a better leaving group.
How do you encourage regio/chemoselectivity of peptide bond formation?
Install appropriate protecting groups.
What groups in an amino acid need protecting?
The N-terminal amine and the C-terminal carboxylic acid.
Any side chain functional groups.
What is the general strategy for dipeptide synthesis?
Where PG = protecting group
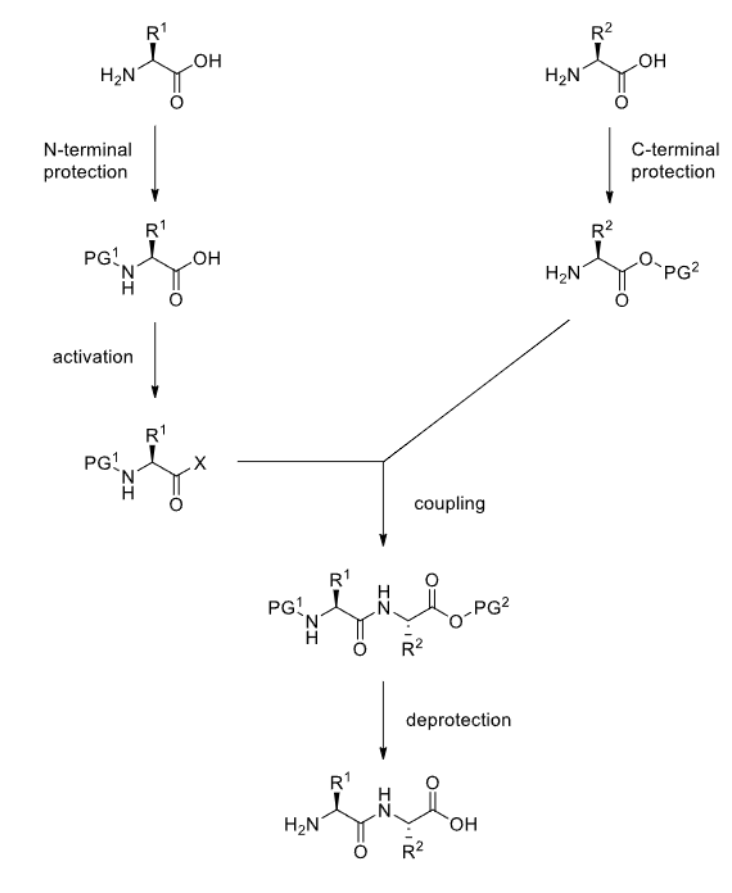
What is a good protecting group for the amine group?
Boc
What is a good protecting group for carboxylic acid groups?
Methyl esters.
How are amino acids activated?
By coupling to tRNA, giving aminoacyl-tRNA molecules.
These are used by ribosomes for protein synthesis.
How is racemisation of amino acids minimised?
A coupling reagent is used, activating the C-terminus.
This generates a less reactive leaving group and allows a one-pot reaction without isolation of the activated species.
Why is peptide synthesis preferred in the C- to N- terminal direction?
The synthesis begins at the C-terminal amino acid and works backwards.
This is due to the risk of oxazolone formation (even with use of a coupling reagent) forming the wrong product.
What is considered to select a suitable protecting group?
Stability - must be stable towards conditions used.
Orthogonality - conditions required for its use must not negatively affect the PGs used.
Selectivity - must be able to selectively install it at specific positions, if needed.
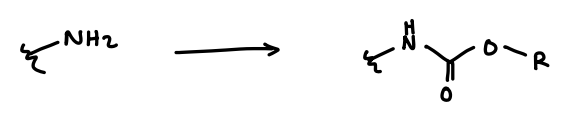
What protecting group is used for amines?
Carbamates.
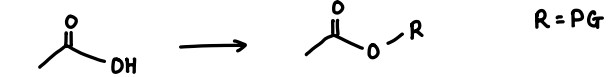
What protecting group is used for carboxylic acids?
Esters.
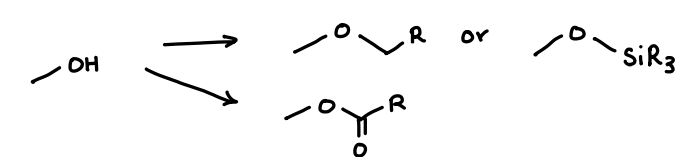
What protecting group is used for hydroxyls?
Alkyl or silyl ethers.
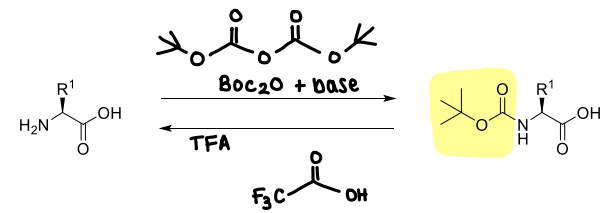
How is Boc used?
It is stable to bases.
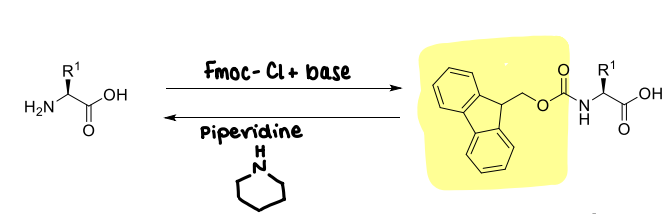
How is Fmoc used?
It is stable to acid.
How are methyl esters used?
They are stable to acids.
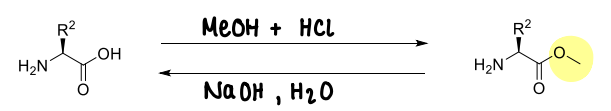
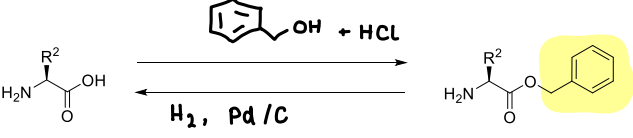
How are benzyl esters made?
It is stable to acids
Why are dimethoxybenzyl groups unstable in acid conditions, but benzyl esters arent?
The dimethoxy groups can contribute to the removal of an OH molecule.
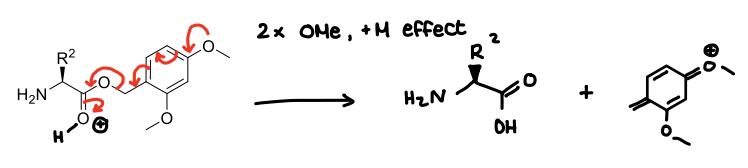
How are tert-butyl esters used?
They are unstable to acids.
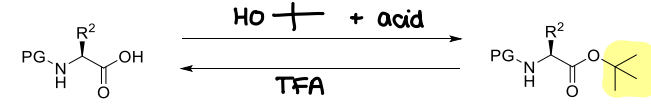
How are tBu ethers used?
Same protection conditions as tBu esters.
Very stable groups and difficult to remove.
Must be cleaved with conc. acid (TFA, HCl)
How are benzyl ethers used?
Its deprotection is similar to benzyl esters.
The N and C terminus are protected to avoid alkylation of the NH2 and COOH.
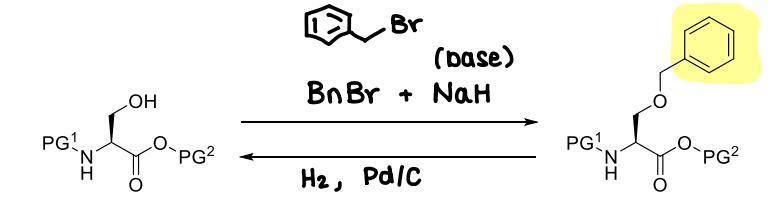
What is PMB?
A variant of the benzyl ether.
It is installed in the same way as benzyl ethers, however must be removed under oxidative cleavage conditions.
How are silyl ethers used?
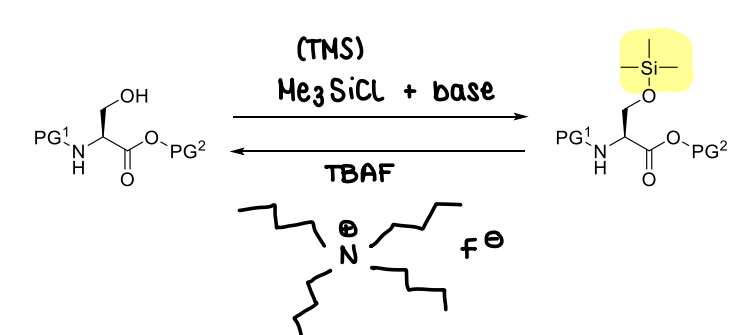
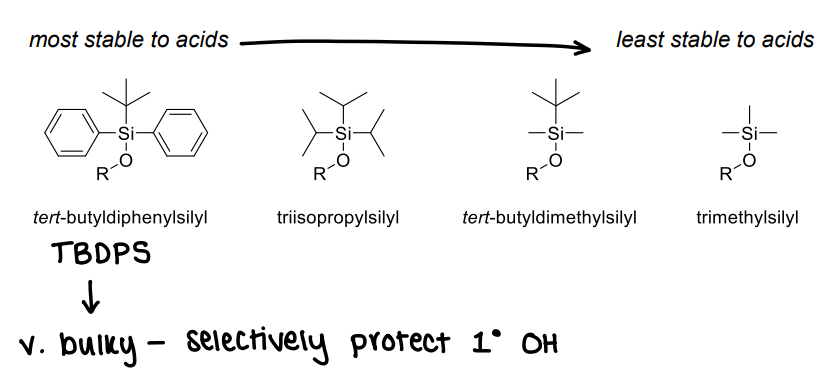
How does the substituent change the stability of silyl ethers?
What is Solid Phase Peptide Synthesis (SPPS)?
When peptides are constructed on insoluble functionalised polymer beads (resin).
How does SPPS work?
The peptide is immobilised on the resin, reagents (in solution) are added, the reaction occurs and then the solvent, unreacted reagent and amino acids are washed away. The product can then be cleaved off once synthesis is complete.

Why does the resin for SPPS need to be functionalised?
The C-terminus of the first amino acid is often used as the site of attachment to the solid support, therefore the resin must be functionalised so that it can be covalently linked the the carboxylate of the amino acid.
What is merrified resin?
Chloromethyl polystyrene.
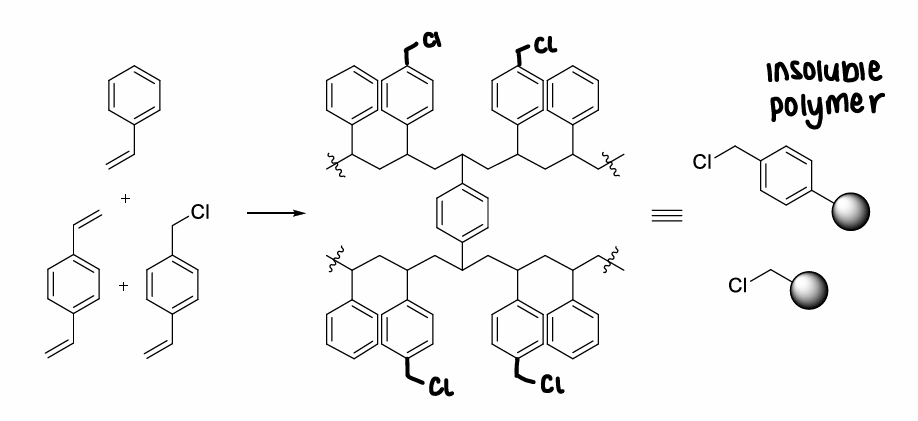
How are amino acids linked onto merrified resin?
Substitution of the chloride leaving group of the resin in an SN2 reaction.
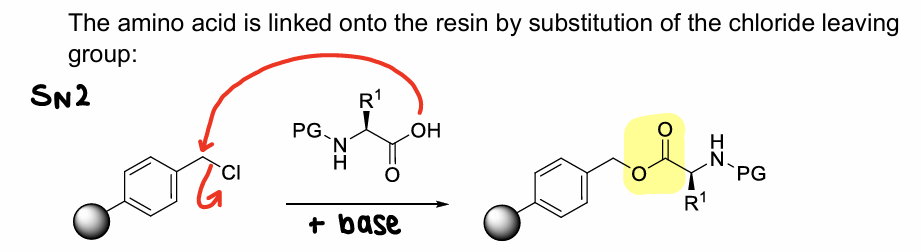
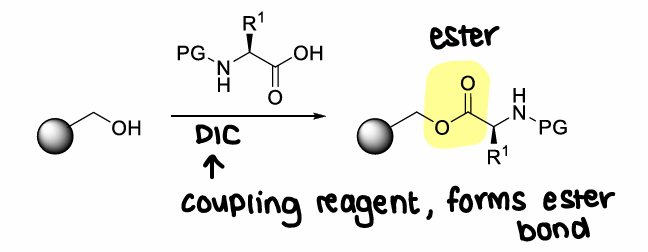
What is wang resin?
A resin with an OH group.
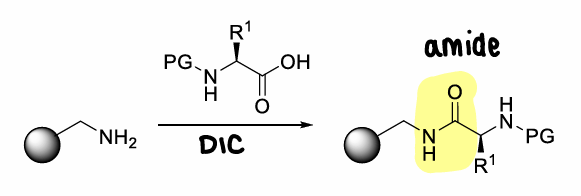
What is a rink amide resin?
A resin with an amine group.
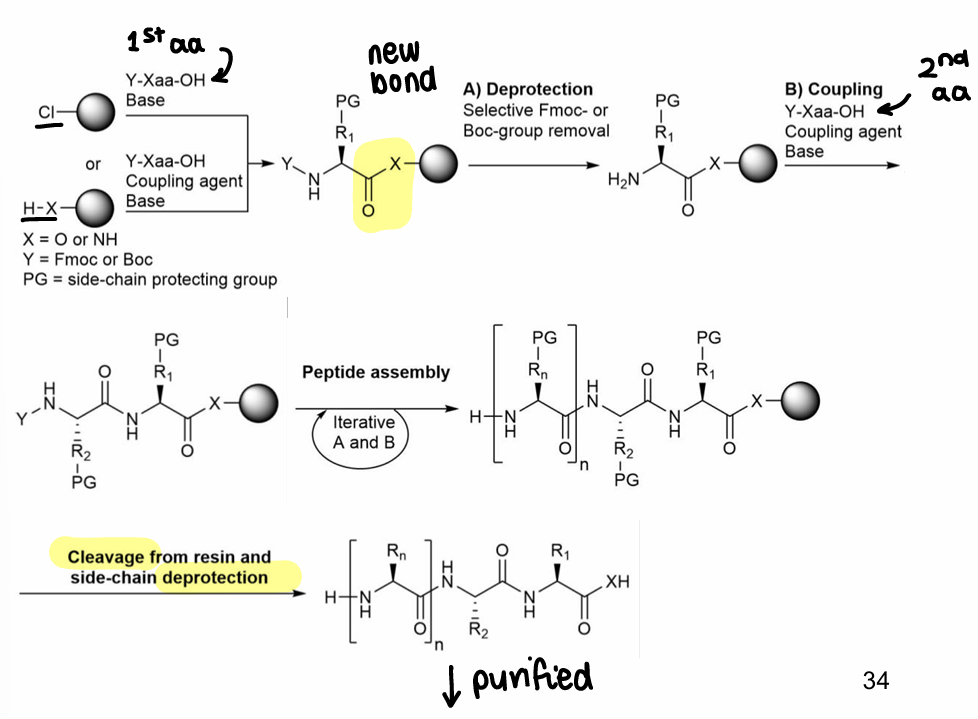
How are peptides synthesised on solid support (using resin)?
First the resin and protected amino acid are bonded.
The amino acid NH is deprotected.
The amino acid is reacted with a second amino acid with a coupling agent.
These steps are repeated, until the peptide is cleaved from the resin and side chains are deprotected, then it is purified.
Why is the N-terminal amine protected with Fmoc, not Boc, in SPPS?
The resins are acid sensitive, so using Fmoc avoids the peptide releasing from the resin when deprotecting the N-terminus.
What coupling reagent is used in SPPS and why?
DIC as it doesnt form insoluble urea byproducts. This byproduct would precipitate out of solution, causing issues in SPPS.
DIC follows the same mechanism as DCC, but the urea byproduct is soluble.
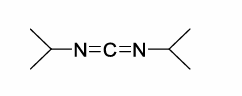
What side chain protecting groups are used in SPPS?
They are chosen so that deprotection occurs simultaneously with release of peptide from the solid support.
The PG must therefore be labile under acidic conditions (Boc, tBu)
How are peptides released from the resin?
Under acidic conditions.
E.g. merrified resin requires corrosive and toxic acid HF.
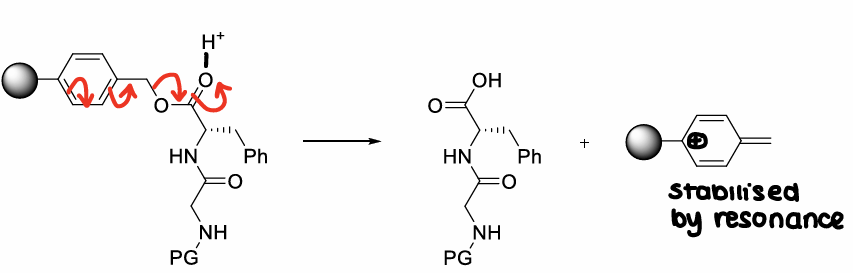
How do you avoid using HF when releasing peptides from resin?
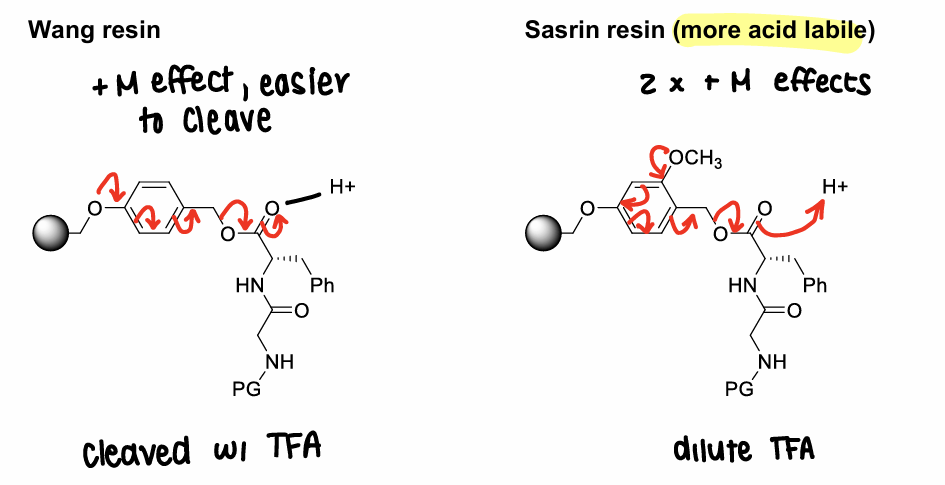
Use other types of resin that contain linkers that allow cleavage under different conditions (usually with a solution of TFA in organic solvent).
E.g. wang resin, sasrin resin
What are the advantages of SPPS?
No work up is required and intermediates dont need to be purified.
It can be automated using an automated peptide synthesiser (would take up to a week manually).
What are the limitations of SPPS?
There can be an accumulation of impurities as no intermediate purification is possible while peptide is bound. (Impurities caused by incomplete coupling reactions).
Purification of the final product can be challenging as SPPS usually leads to complex mixtures of products.
A limited amount of peptide can be produced.
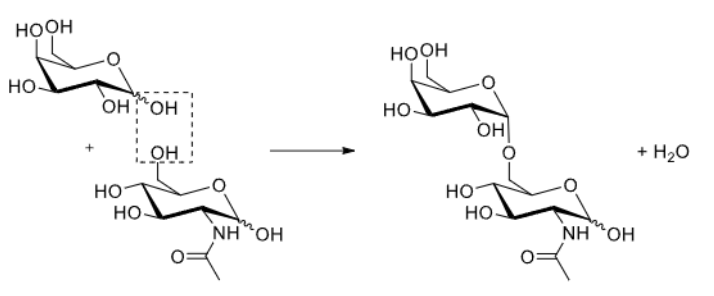
How do two glucose residues react?
Forms a 1,3 beta glycosidic bond.
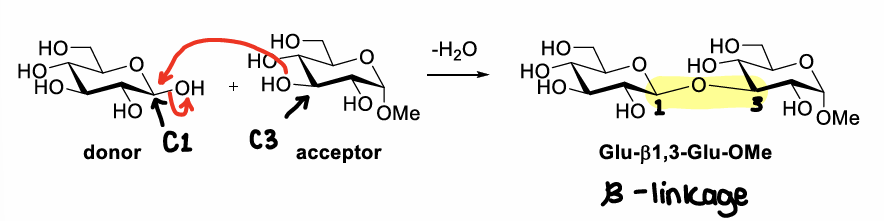
Why is the reaction of two glucose molecules challenging?
The OH- is a poor leaving group, so reaction is inefficient.
Regioselectivity is challenging as there are many hydroxyl groups.
Stereoselectivity is also challenging as alpha or beta anomers could be made.
How do you achieve efficient and selective coupling between two monosaccharides?
Increase donor reactivity by activating the anomeric hydroxyl group (turn into better LG).
Install appropriate PGs to accomplish regioselectivity.
Select appropriate reaction conditions and PGs for stereoselectivity.
When is the beta anomer favoured?
If the PG on the donor is an ester.
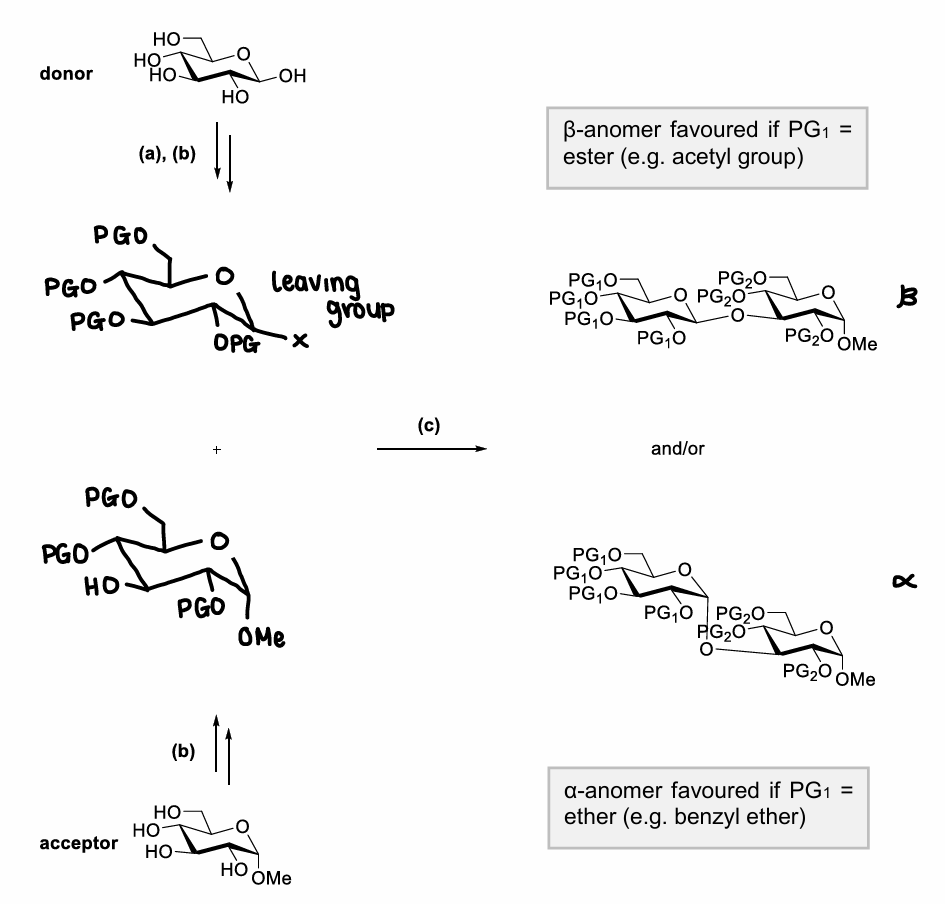
When is the alpha anomer favoured?
If the PG on the donor is an ether.
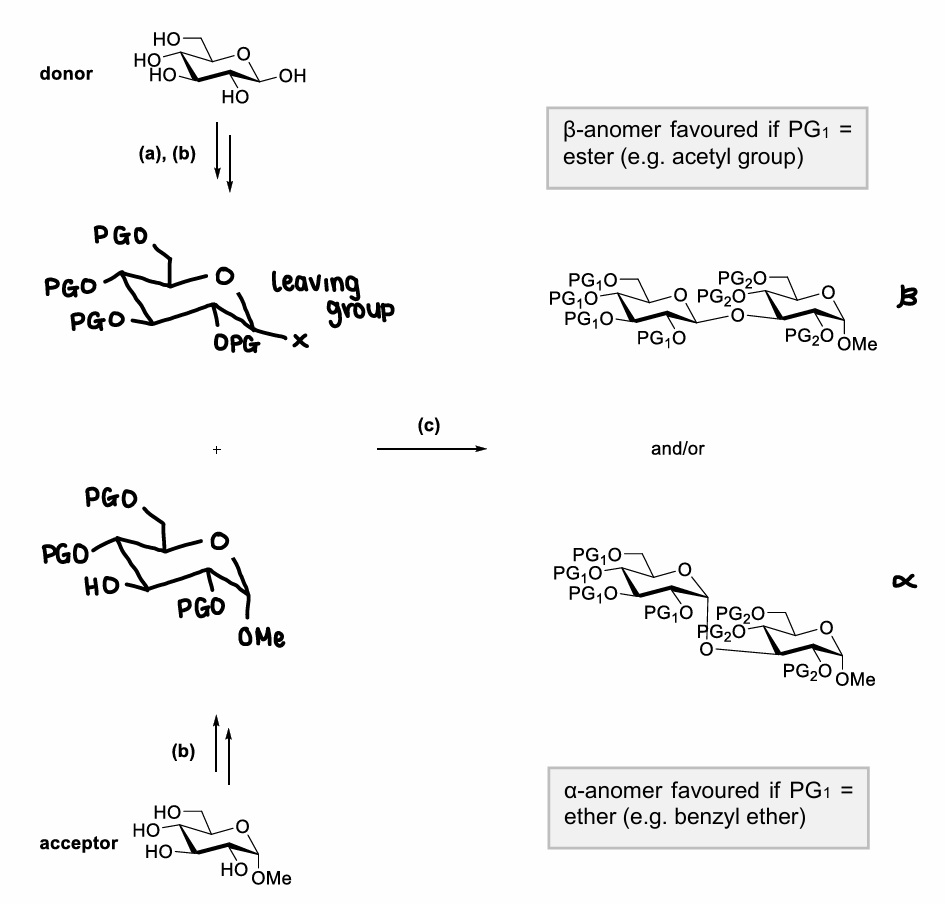
Which OH groups on the monosaccharide are most/least reactive?
The primary OH is most reactive.
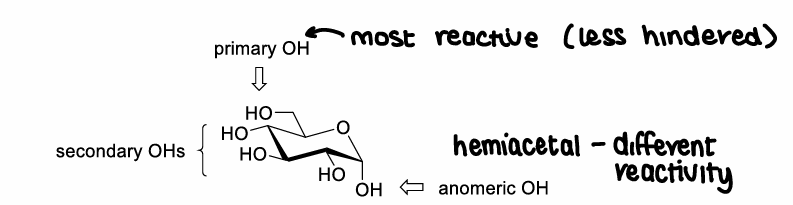
How do you selective protect the primary OH group?
Can use the higher reactivity of the primary OH.
Can use PGs that are very sterically demanding that will add to the least hindered OH.
How does trityl protect the primary OH?
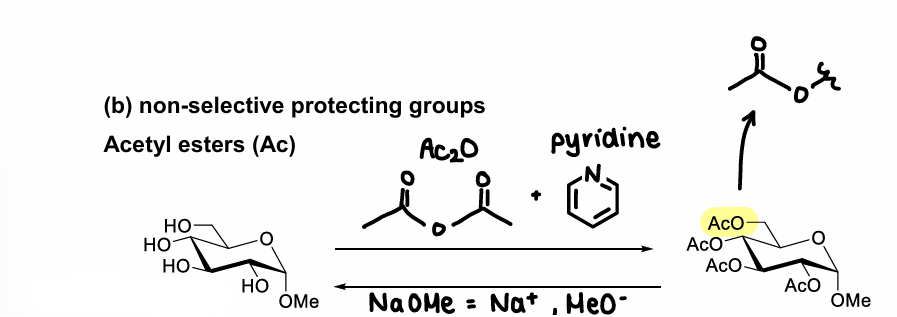
How are non-selective PGs used to protect monosaccharides?
They protect every OH group, leaving only the OMe group (which is required for attack).
How do acetyl ester protect OH groups?
Turns OH into AcO.
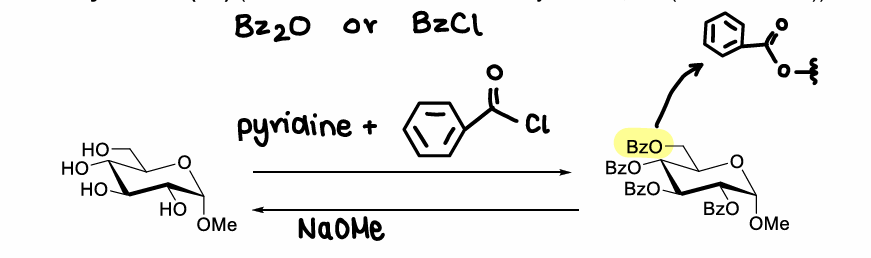
How do benzoyl esters protect OH groups?
What is Fisher glycosylation?
The production of an alkyl glycoside.
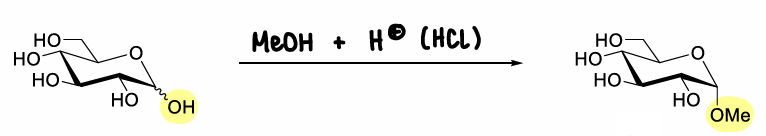
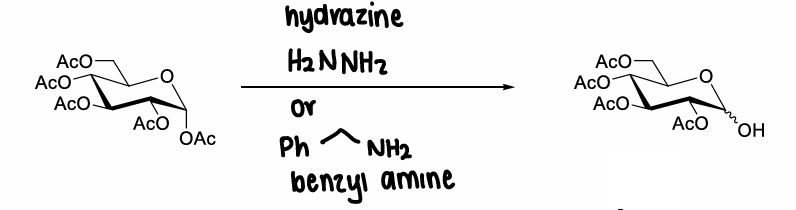
How are anomeric acetyl esters deprotected?
How are 1,3 diols selectively protected?
Using a PG that can bridge over two neighbouring OH groups, forming an acetal.
How do benzylidene acetals protect diols?
It prefers a 1,3 diol over a 1,2 diol, so adds onto OHs at C4 and C6.
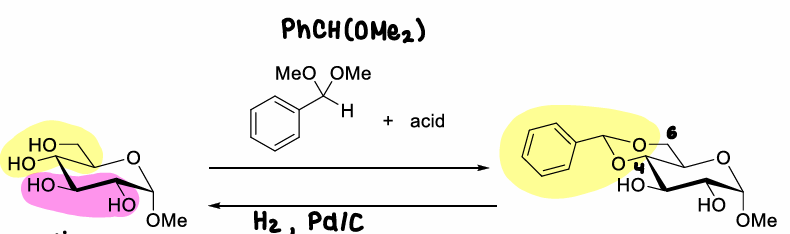
How does selective cleavage of the benzylidene acetal form the OH groups?
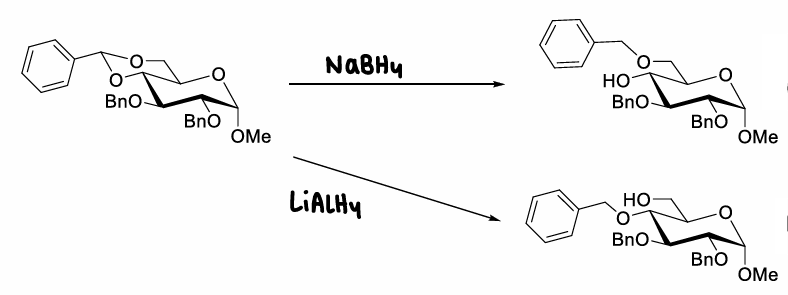
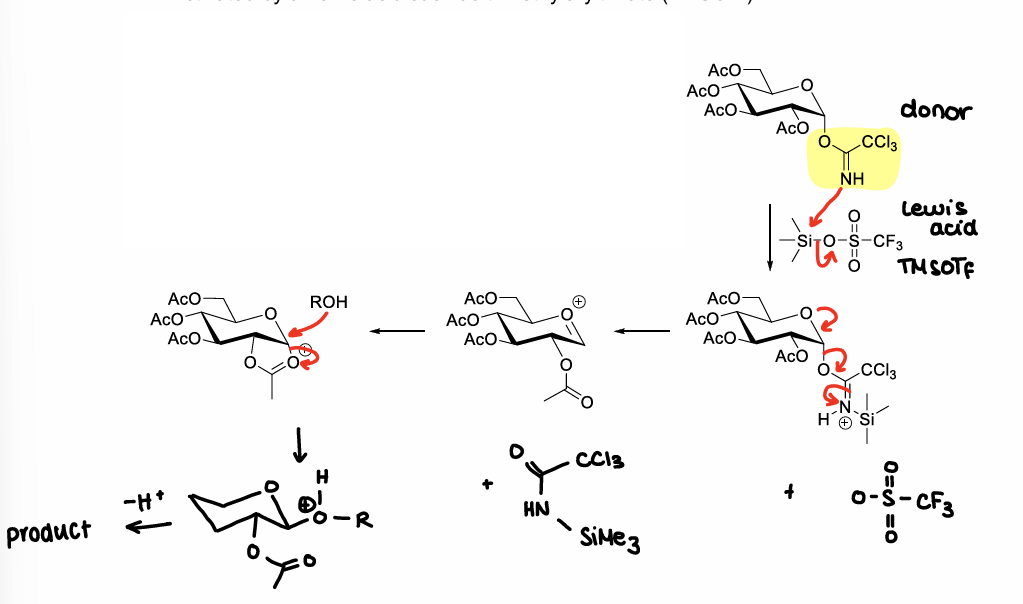
How is the anomeric site activated?
Install a good leaving group at the anomeric position of the glycosylation donor, therefore enabling reaction with an acceptor molecule (activation). This forms an oxocarbenium ion.

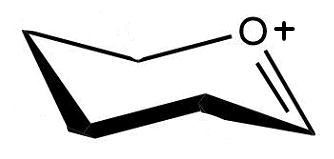
How can an oxocarbenium ion be attacked by an acceptor?
Can be attacked from either face of the ring, therefore forming either alpha or beta anomers.
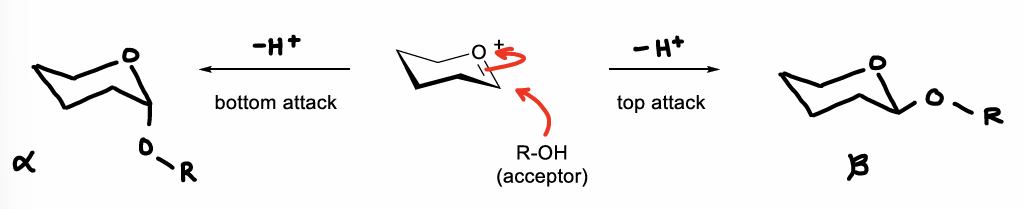
How is a transition state with partial oxocarbenium ion character formed?
The leaving group may not fully dissociate.
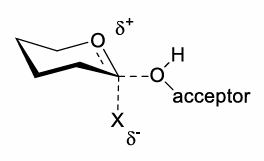
What can be used as a leaving group when trying to form an oxocarbenium ion?
Glycosyl bromide (activated by silver salt)
Trichloroacetimidate (activated by Lewis acid)
Thioethers
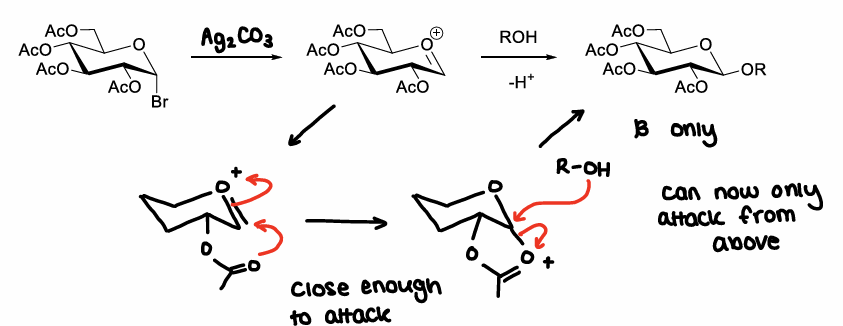
How can the stereochemical outcome of the glycosylation of glycosyl bromide reaction be controlled?
Depends on the protecting group at the C2 position. If it is close enough to attack the oxocarbenium ion it may prevent attack from below the ring, therefore only beta anomers are made.
How are trichloroacetimidate molecules formed?
Using CCl3CN + NaH
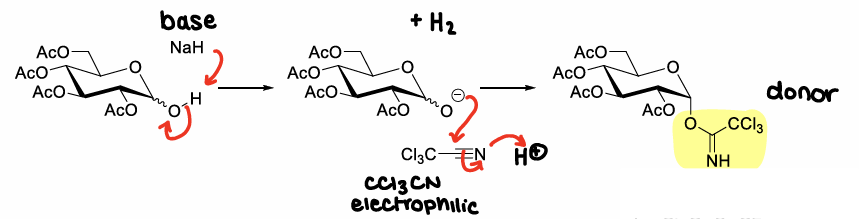
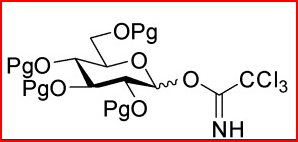
How are trichloroacetimidate molecules activated?
Using Lewis acids, e.g. TMSOTf
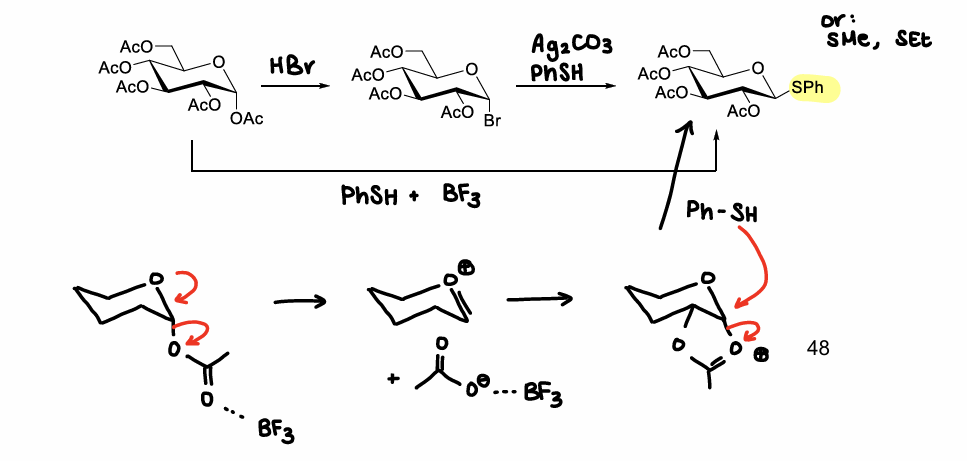
How are thioether glucose molecules formed?
Using PhSH & BF3, or HBr then Ag2CO3 & PhSH
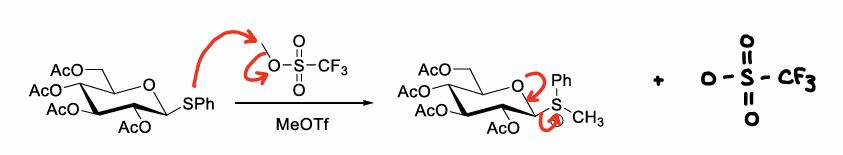
How are thioethers further made into good leaving groups?
Further derivatisation:
Iodination
Methylation
Oxidation
How are thioethers iodinated?
Using N-iodosuccinimide + acid

How are thioethers methylated?
Using methyltriflate or TMSOTf.
How are thioethers oxidised?
Using mCPBA, then follow with Tf2O.
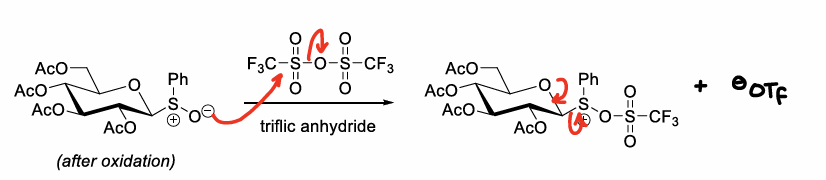
What is the anomeric effect?
A main driver of the stereochemical outcome, causing the alpha anomer to be the major product.
How is the anomeric effect explained by dipole minimisation?
In the beta anomer, the dipoles of the endocyclic oxygen and the anomeric oxygen are partially aligned, giving unfavourable repulsion.

How is the anomeric effect explained by hyperconjugation?
In the alpha anomer, there is orbital overlap between the axial lone electron pair on the endocyclic oxygen and the antibonding orbital of the axial C-O bond. This makes a stabilising interaction, so the molecules energy is lowered.

What is the neighbouring group participation effect?
When there is a protecting group at the C2 position, a bicyclic oxonium ion intermediate can be formed after elimination of the anomeric leaving group.
This sterically hinders attacks from one side of the ring, leading to one isomer forming.
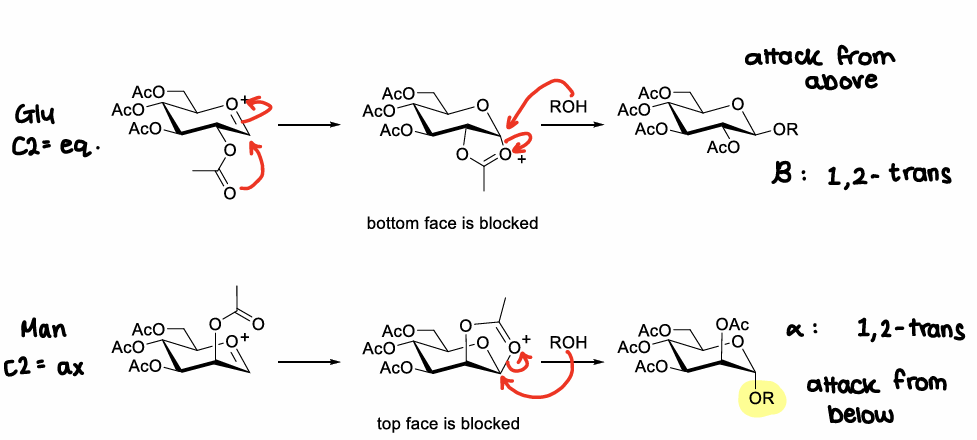
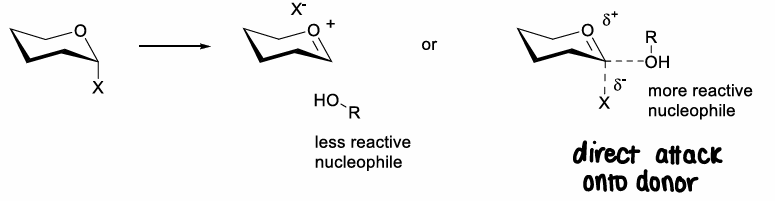
How does the reactivity of the acceptor affect the stereochemical outcome of glycosylation?
Via conformation or steric effects.
If there is a more reactive nucleophile, it will go against the anomeric effect as they can go via an SN2 type reaction, in which the oxocarbenium ion does not fully form so the anomeric effect doesnt play a role. Inversion of the stereochemistry occurs.
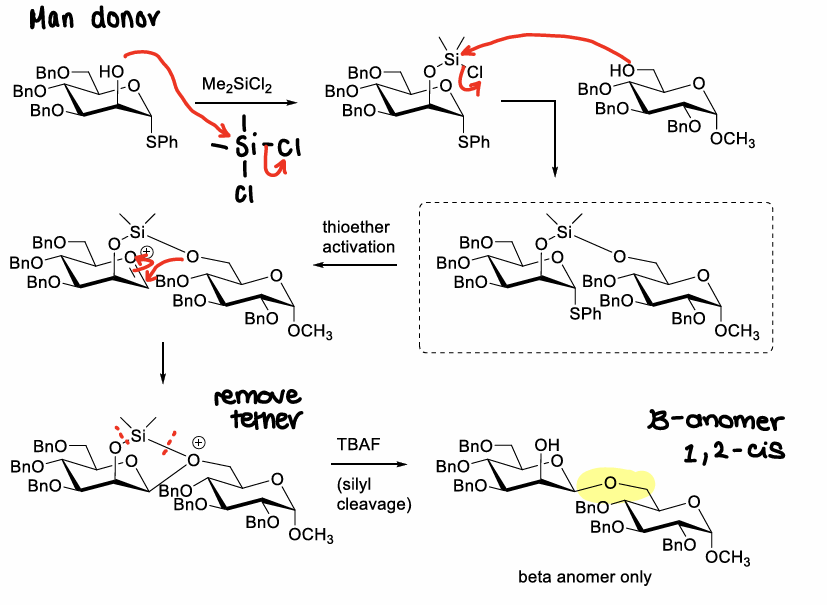
What is intramolecular tethering?
When a temporary linker between an acceptor and donor holds the acceptor in place to preferentially attack the donor from one face of the ring.
How does intramolecular tethering work?
Usually a mannose donor is used.