Chem 201: Ch 7 - Molecular Geometry, Intermolecular Forces, and Bonding Theories
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
Molecular shape can be predicted by using the
valence-shell electron-pair repulsion (VSEPR) model.
ABx
A is the central atom surrounded by x B atoms.
x can have integer values of 2 to 6.
The basis of the VSEPR model is that
electrons repel each other.
Electrons are found in various domains.
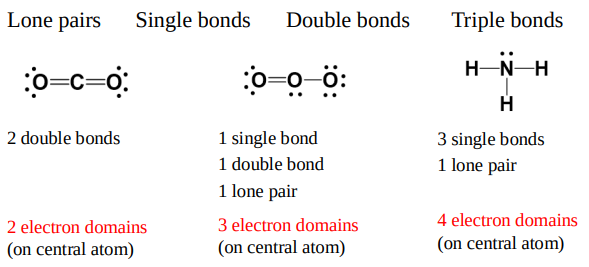
Electrons will arrange themselves to be
as far apart as possible.
Arrangements minimize repulsive interactions.
The electron domain geometry
is the arrangement of electron domains around the central atom
The molecular geometry
is the arrangement of bonded atoms
In an ABx molecule
a bond angle is the angle between two adjacent A–B bonds.
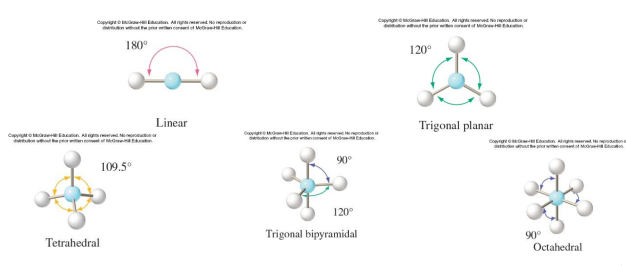
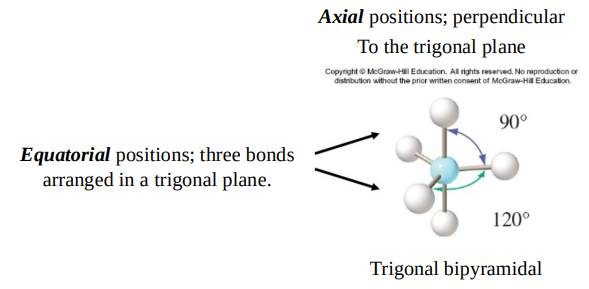
AB5 molecules contain two different bond angles between adjacent bonds.
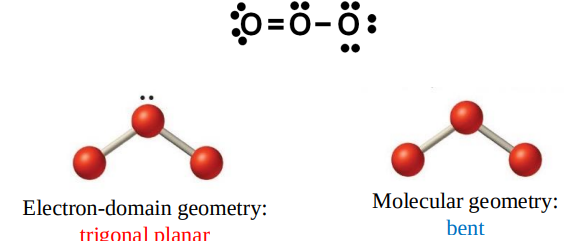
When the central atom in an ABx molecule bears one or more lone pairs,
the electron-domain geometry and the molecular geometry are no longer the same.
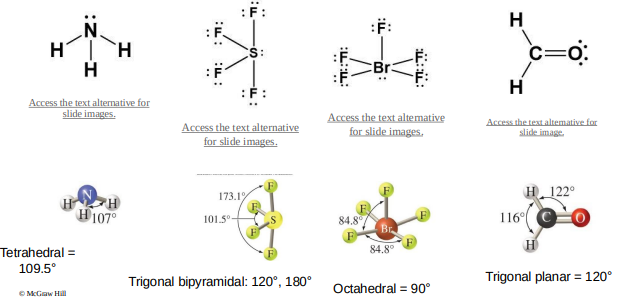
The steps to determine the electron-domain and molecular geometries are as follows:
Step 1: Draw the Lewis structure of the molecule or polyatomic ion.
Step 2: Count the number of electron domains on the central atom.
Step 3: Determine the electron-domain geometry by applying the VSEPR model.
Step 4: Determine the molecular geometry by considering the positions of the atoms only.
Some electron domains are better than others at repelling neighboring domains.
1. Lone pairs take up more space than bonded pairs of electrons.
2. Multiple bonds repel more strongly than single bonds.
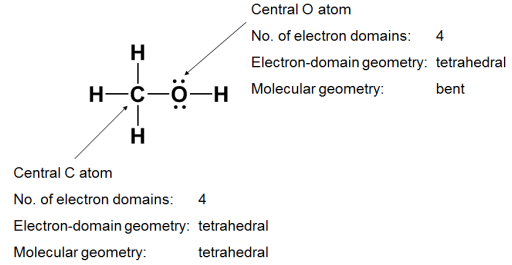
The geometry of more complex molecules can be determined by treating them as though they have multiple central atoms.
Molecular polarity is one of the most important consequences of molecular geometry.
A diatomic molecule is polar when the electronegativities of the two atoms are different.
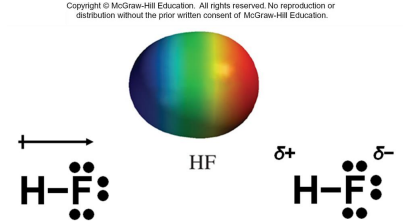
The polarity of a molecule made up of three or more atoms depends on:
1. The polarity of the individual bonds.
2. The molecular geometry.
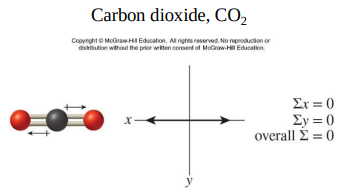
The bonds in CO2 are polar but the molecule is nonpolar.
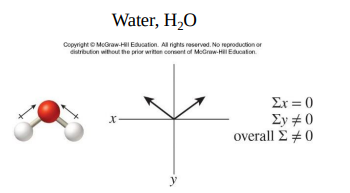
The bonds in H2O are polar and the molecule is polar.
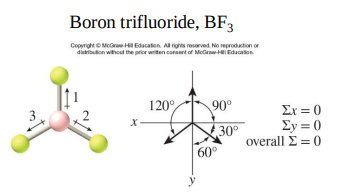
The bonds in BF3 are polar but the molecule is nonpolar.
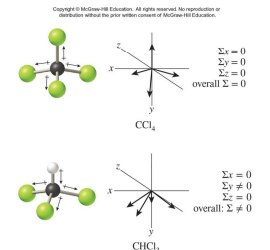
The bonds in CCl4 are polar but the molecule is nonpolar.
The bonds in CHCl3 are polar and the molecule is polar.
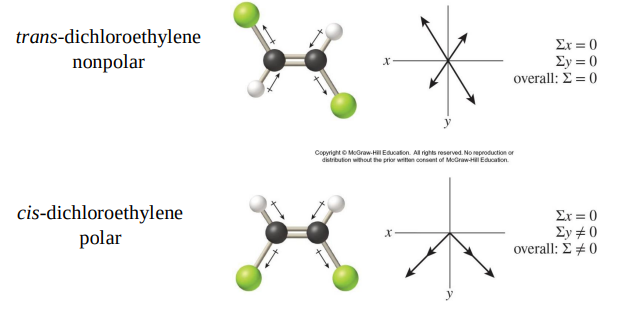
Dipole moments can be used to distinguish between structural isomers.
Intermolecular forces are
attractive forces between neighboring molecules.
These forces are known collectively as van der Waals forces.
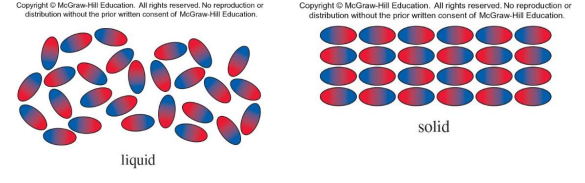
• The magnitude (and type) of intermolecular forces can be sufficient to hold the molecules of a substance together in a solid or liquid.
• Gases have no apparent intermolecular forces.
Dipole–dipole interactions are
attractive forces that act between polar molecules.
The magnitude of the attractive forces depends on the magnitude of the dipole.
• Red is high electron density (δ−) and blue is low (δ+)
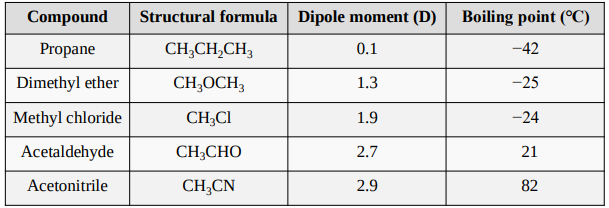
Dipole Moments and Boiling Points of Compounds with Similar Molecular Masses
Hydrogen bonding
is a special type of dipole–dipole interaction.
Hydrogen bonding only occurs in molecules that contain H bonded to a small, highly electronegative atom such as N, O, or F
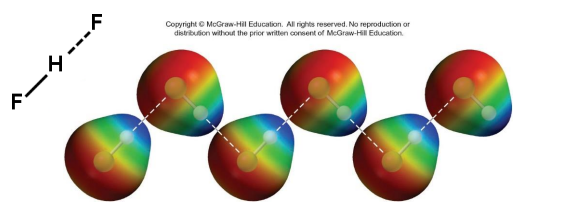
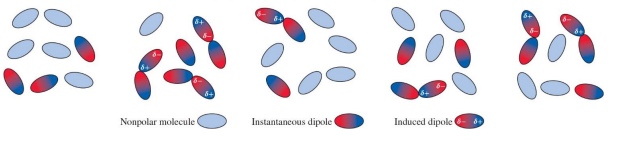
Dispersion forces
or London dispersion forces result from the Coulombic attractions between instantaneous dipoles of nonpolar molecules.
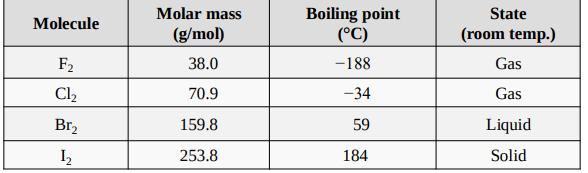
Molar Masses, Boiling Points, and States of the Halogens at Room Temperature
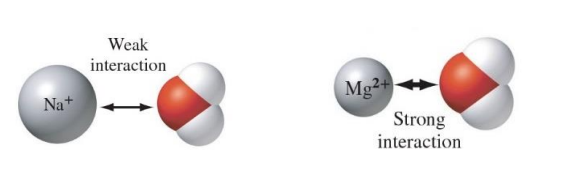
Ion–dipole interactions
are Coulombic attractions between ions (either positive or negative) and polar molecules.
According to valence bond theory, atoms share electrons when atomic orbitals overlap.
1. A bond forms when single occupied atomic orbitals on two atoms overlap.
2. The two electrons shared in the region of orbital overlap must be of opposite spin.
3. Formation of a bond results in a lower potential energy for the system
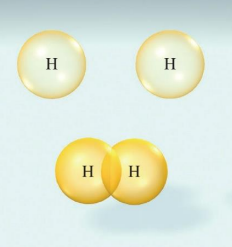
The H–H bond in H2 forms when the singly occupied 1s orbitals of the two H atoms overlap:
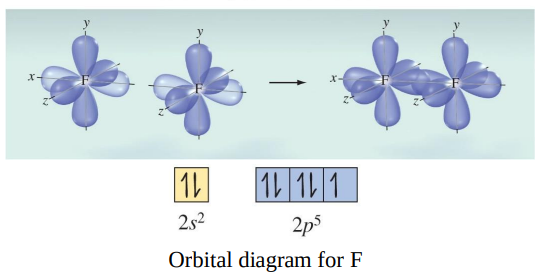
The F−F bond in F2 forms when the singly occupied 2p orbitals of the two F atoms overlap:
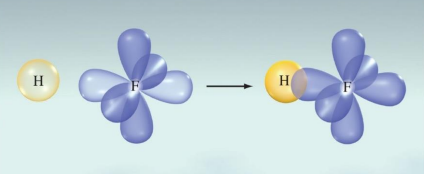
The H−F bond in HF forms when the singly occupied 1s orbital on the H atom overlaps with the single occupied 2p orbital of the F atom:
This overlap creates a polar covalent bond, allowing for the sharing of electrons between hydrogen and fluorine.
Valence Bond Theory 5
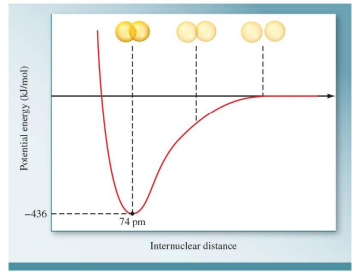
A covalent bond will form if the potential energy of the molecule is lower than the combined potential energies of the isolated atoms.
Hybridization
or mixing of atomic orbitals can account for observed bond angles in molecules that could not be described by the direct overlap of atomic orbitals.
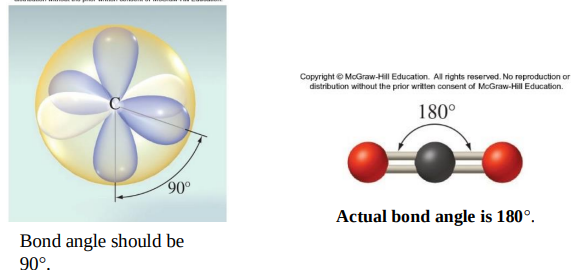
BeCl2 Lewis theory and VSEPR theory predict Cl−Be−Cl bond angle of 180°.
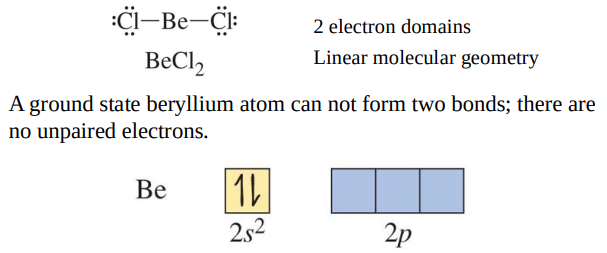
An excited state configuration for Be has two unpaired electrons and can form to bonds.
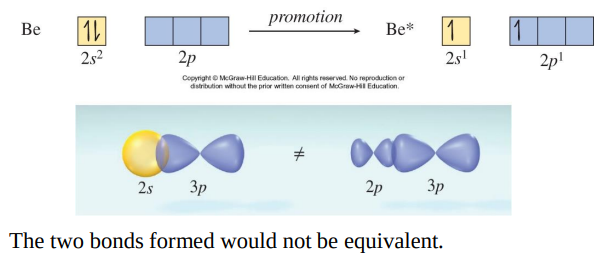
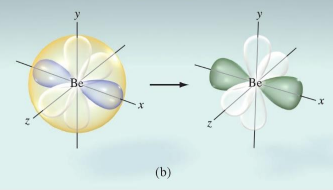
Experimentally the bond in BeCl2 bonds is identical in length and strength. Mixing of one s orbital and one p orbital to yield two sp orbitals.
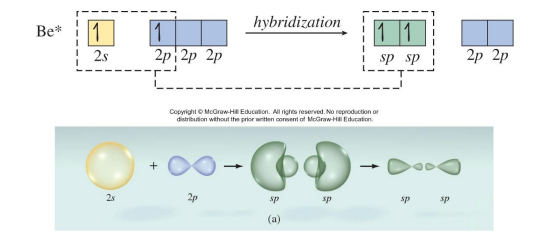
The 2s orbital and one of the 2p orbitals on Be combine to form two sp hybrid orbitals.
Like any two electron domains, the hybrid orbitals on Be are 180° apart.
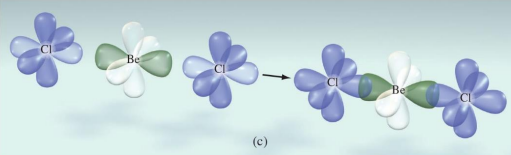
The hybrid orbitals on Be each overlap with a singly occupied 3p orbital on a Cl atom
The energy required to form an excited state Be atom is more than compensated for by the energy given off when a bond forms.
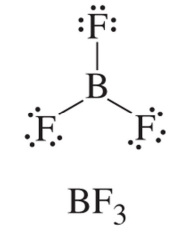
Determine the number and type of hybrid orbitals necessary to rationalize the bonding in BF3.
Step 1: Draw the Lewis structure:
Step 2:
Count the number of electron domains on the central atom. This is the number of hybrid orbitals necessary to account for the molecule’s geometry. (This is also the number of atomic orbitals that must undergo hybridization.)

Step 3:
Draw the ground-state orbital diagram for the central atom
Step 4:
Maximize the number of unpaired valence electrons by promotion.

Step 5:
Combine the necessary number of atomic orbitals to generate the required number of hybrid orbitals.
Step 6:
Place electrons in the hybrid orbitals, putting one electron in each orbital before pairing any electrons.