Electrorefining
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
draw it
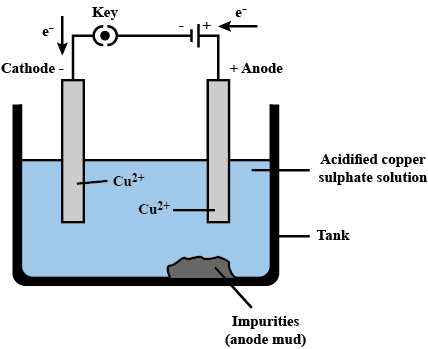
Process
The impure metal to be purified is made the anode (positive electrode).
A thin sheet of the pure metal is used as the cathode (negative electrode).
The anode and cathode are immersed in an electrolyte solution, typically an aqueous solution containing ions of the metal being refined.
When an external electrical potential is applied the rxns happen
The pure metal progressively gets deposited in a thin layer on the cathode, while the anode is consumed over time.
The electrolyte solution is replenished with metal ions as the anode dissolves to maintain the concentration.
Equations
At the Anode (Oxidation):
The impure metal is oxidized, going into the electrolyte solution as positively charged metal ions.
For a metal M: M(s) → M^(n+) + ne^-
At the Cathode (Reduction):
The pure metal ions from the electrolyte are reduced and deposited as pure metal on the cathode.
For a metal M: M^(n+) + ne^- → M(s)
Common impurities etc
Common Impurities
Au= -1.5V
Ag= -0.8V
Pt= really (-)
Mg = 2.36V
Zn = 0.76V
Al= 1.68V
To ensure these metals do not reduce onto the cathode we control the voltage based on the E° value
Gold, silver, platinum
We cannot allow these ions to oxidise because if they did they will naturally reduce to the high reductive energy therefore keep V at 0.34V
As the blister copper loses mass the precious metals fall to the bottom in anode mud
Magnesium zinc and aluminium
Conversely these metals will naturally oxidise into solution however by maintaining a small voltage we can stop these from reducing