Exothermic and Endothermic reactions.
1/8
Earn XP
Description and Tags
https://www.youtube.com/watch?v=4HS6D0hTzdg&list=PL9IouNCPbCxX74bPfz0TGVVmyGYgMarWu
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Whats an exothermic reaction
They transfer energy from the reacting molecules to the surroundings. This means in exothermic reactions the temperature of the surroundings increase.
Combustion is an exothermic reaction
What does an energy profile dagram do
Scientists represent the energy changes that take place in reactions by drawing an energy profile diagram
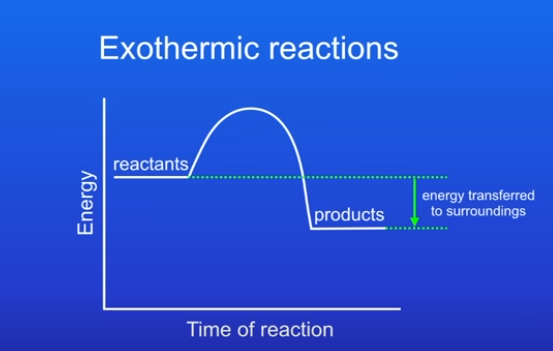
What does energy profile diagram for exothermic reaction look lke
The products have less energy than reactants, this is because energy has been transferred from reaction to the surroundings.
The difference between the energy of reactants and energy of products tells us energy that has been released to the surroundings.
Products below reactants
What are uses of exothermic reactions
Hand warmers
Self heating cans eg for food or drink.
Whats endothermic reaction
They take in energy from their surroundings. So the temperature of the surroundings decreasse.
e.g thermal decomposition
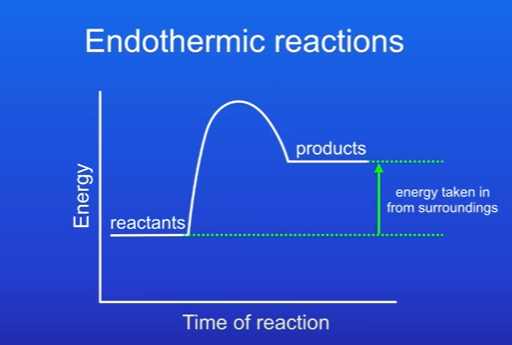
What does energy profile diagram of endothermic reaction look like
The products have more energy than the reactants, this is because energy has been taken in from the surroundings.
The difference in energy from reactants and products tells us amount of energy that has been taken in by the reaction
Products above reactants.
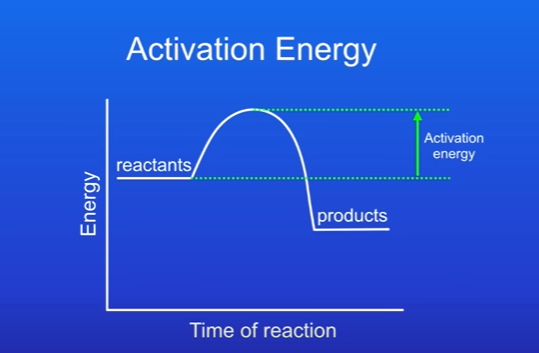
Whats the activation energy
When the energy in diagram rises to a peak.
The minimum amount of energy that particles must have in order to react is called the activation energy. This is because reactions only occur when particles collide w each other.
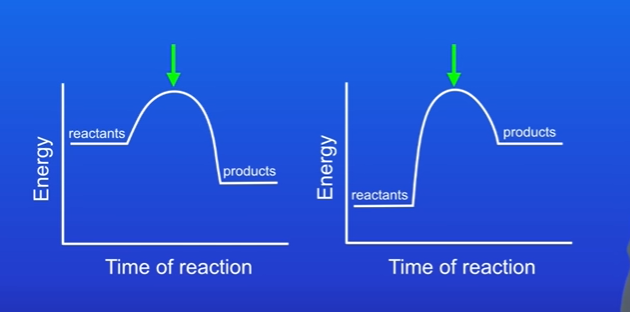
What does activation energy look like on endothermic reaction
Lots of energy needed.
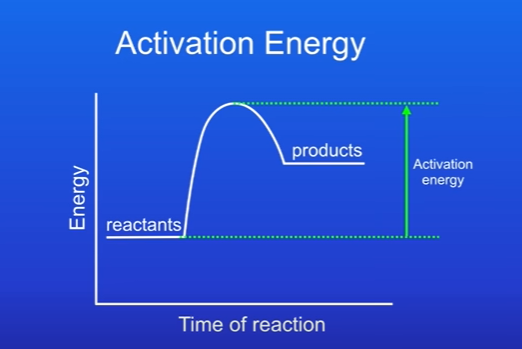
What does activation energy look like on exothermic reaction
Not much needed.