chemistry - key concepts in chemistry: calculations involving masses (1.43 - 1.53)
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
notations
Mr = relative formula mass
Ar = relative atomic mass
1.43 calculate Mr given Ars
Mr = sum of Ar of atoms in formula
e.g. Mr of CO2:
Ar of C = 12, Ar of O = 16
12 + (2 × 16) = 44
1.43 calculate % by mass of element in compound given Ars
e.g. % by mass of oxygen in CO2:
Mr of CO2 = 12 + (2 × 16) = 44
O2 = 2 × 16 = 32
oxygen/total = 32/44 = 0.727 × 100% = 72.7%
empirical formula
simplest whole number ratio of atoms of each element in substance
molecular formula
actual number of atoms of each element in one molecule of substance
1.44 calculate formulae of simple compounds (empirical formulae) from reacting masses
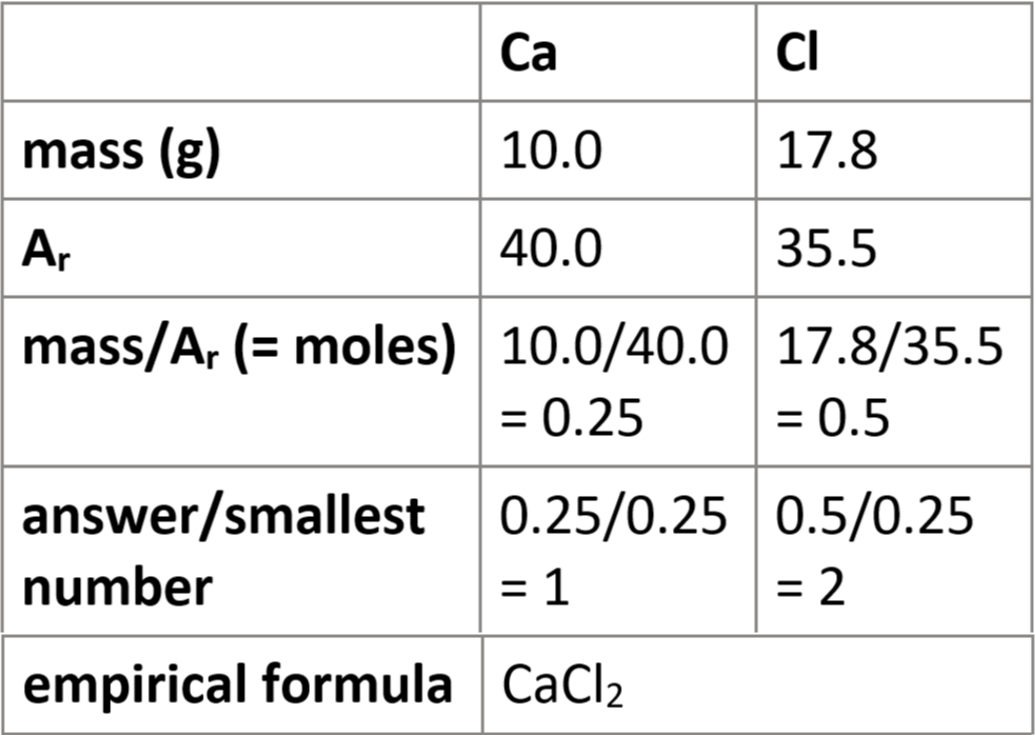
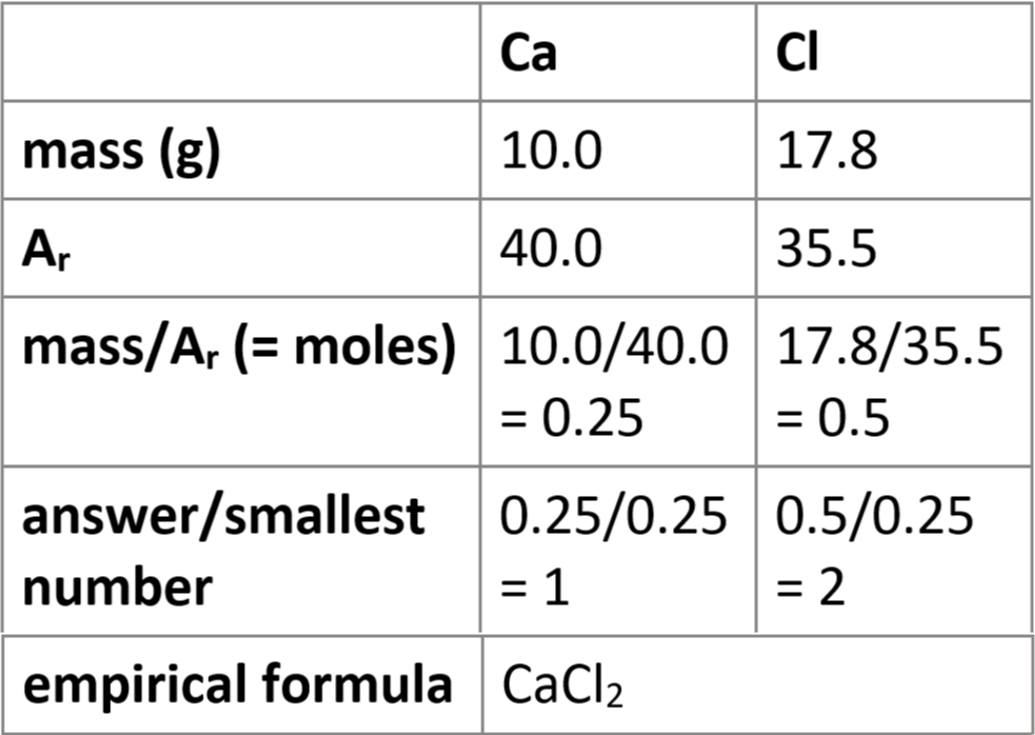
1.44 calculate formulae of simple compounds (empirical formulae) from % composition
assume 100g sample
convert % → grams
e.g. 75% = 75g
follow table
1.45 find empirical formula from molecular formula
e.g. empirical formula of C6H12O6:
find HCF = 6
divide subscripts by 6: 6/6 = 1; 12/6 = 2
CH2O
1.45 find molecular formula from empirical formula & Mr
e.g. molecular formula of glucose: empirical formula = CH2O, Mr = 180
find empirical formula mass: 12 + (2 × 1) + 16 = 30
Ar of C = 12, Ar of H = 1, Ar of O = 16
Mr/empirical formula mass: 180/30 = 6
molecular formula = empirical formula x 6: C6H12O6
1.46 how to find empirical formula of simple compound - e.g. magnesium oxide
heat magnesium ribbon in limited oxygen supply
weigh reactant & product
e.g. empirical formula of MgO: 0.576g Mg ribbon heated, produced 0.960g MgO
find mass O: 0.960 - 0.576 = 0.384
O = 0.384, Mg = 0.576
answer/smallest number: 0.384/0.384 = 1, 0.576/0.384 = 1.5
make whole number ratio: 1:1.5 = 2:3
empirical formula = Mg2O3
1.47 law of conservation of mass in closed system - precipitation reaction
lead nitrate solution + potassium iodide solution → lead iodide (yellow precipitate) + potassium nitrate (colourless solution)
Pb(NO3)2 (aq) + 2KI (aq) → PbI2 (s) + 2KNO3 (aq)
number of atoms doesn’t change - mass can’t change
closed system - no new substances added/removed
1.47 law of conservation of mass in non-enclosed system - open flask
copper carbonate heated in air → copper oxide + carbon dioxide
CuCO3 (s) → CuO (s) + CO2 (g)
mass of copper oxide left < mass copper carbonate at start
CO2 gas escapes - mass decreases
non-enclosed system - gas can escape
1.48 calculate masses of reactants & products from balanced equations given mass of 1 substance
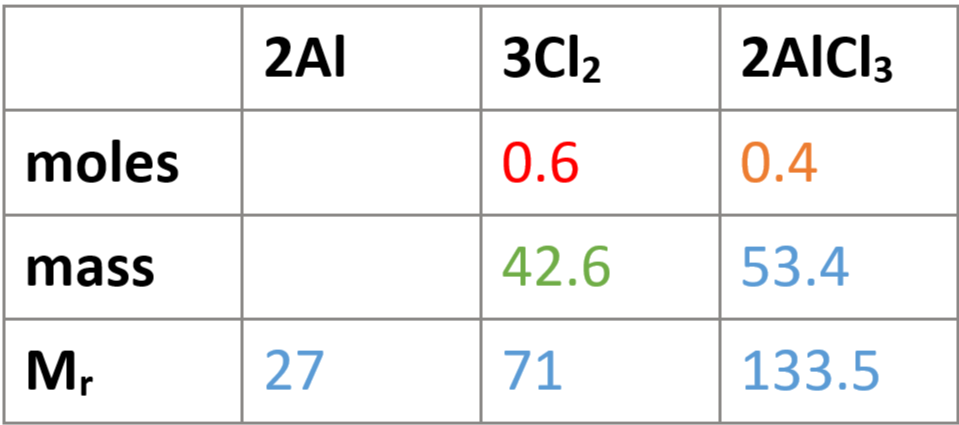
e.g. mass of chlorine needed to make 53.4g of aluminium chloride
write balanced equation: 2Al + 3Cl2 → 2AlCl3
fill in Mrs & known masses in table
Mr doesn’t take big numbers into account
find moles of aluminium chloride: n = m/Mr → 53.4/133.5 = 0.4
find moles of chlorine: 3:2 = x:0.4, 2/0.4 = 5, 3/5 = 0.6, x = 0.6 → 3:2 = 0.6:0.4
find mass of chorine: m = n x Mr → 0.6 × 71 = 42.6g
1.49 calculate concentrations of solutions in g dm-3
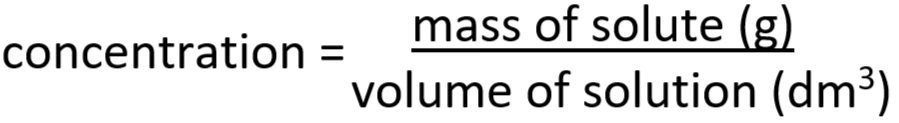
1.50 Avogadro constant
one mole of particles of substance = 6.02 × 1023 atoms/ molecules/ions
1.50 moles & mass
mass of one mole of substance = Ar or Mr in grams
e.g. mass of one mole of O2 = 16 × 2 = 32g
1.51 calculate number of moles in given mass
mass/Mr = number of moles
1.51 calculate mass given number of moles
moles × Mr = mass
1.51 calculate number of particles given number of moles
number of moles × (6.02 × 1023) = number of particles
1.51 calculate number of moles given number of particles
number of particles/(6.02 × 1023) = number of moles
1.51 calculate number of particles given mass
mass/Mr = moles
number of moles × (6.02 × 1023) = number of particles
1.51 calculative mass given number of particles
number of particles/(6.02 × 1023) = number of moles
moles × Mr = mass
1.52 mass of product formed controlled
one of reactants often added in excess - not completely used up
mass of product formed controlled by mass of reactant not in excess (limiting reactant)
stoichiometry
ratio of moles of each substance
1.53 find stoichiometry of from masses of reactants & products (finding balanced equation)
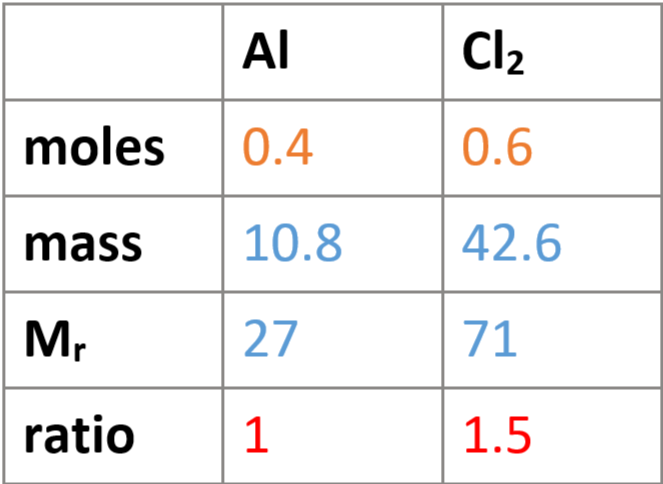
e.g. 10.8g of aluminium reacted with 42.6g of chlorine to produce aluminium chloride (AlCl3) - find balanced equation:
fill in Mrs & known masses in table
find moles aluminium & chlorine: n = m/Mr → 10.8/27 = 0.4, 42.6/71 = 0.6
divide both answers by smaller number: 0.4/0.4 = 1, 0.6/0.4 = 1.5
find simplest whole number ratio & write equation: 1:1.5 = 2:3 → 2Al + 3Cl2 = xAlCl3
balance normal way → 2Al + 3Cl2 = 2AlCl3